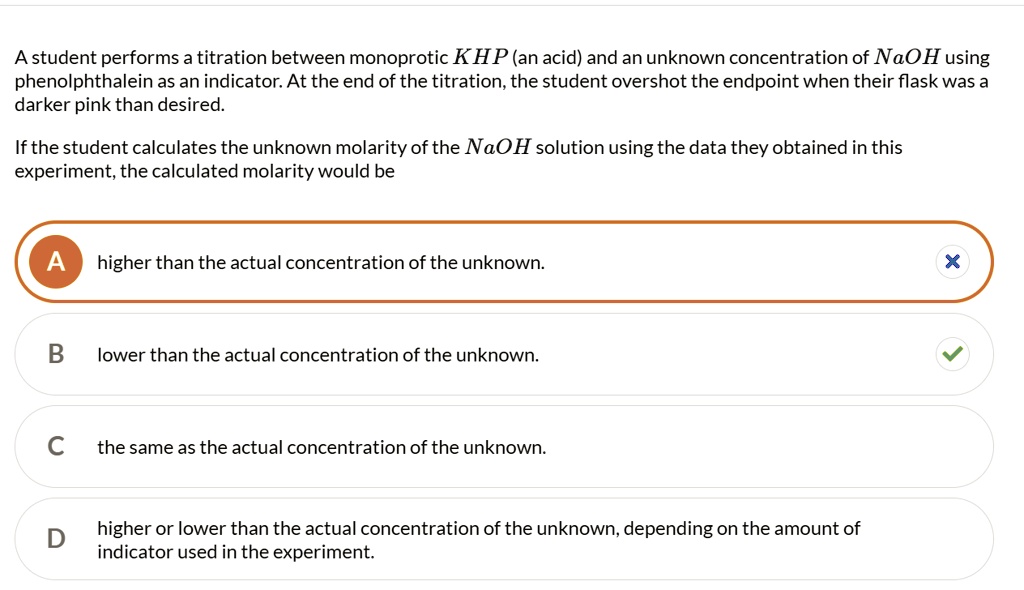
the endpoint of the titration is overshot in stock
You suspect that you overshot the endpoint of the titration (or added too much NaOH solution in your first attempt at titrating the KHP with NaOH.) Since the calculations are simple you go ahead and calculate the concentration of NaOH for this first titration along with the values for later titrations. When the concentration of your suspect (overshot) value is compared to the concentration values of the rest of your titrations what would you look for in the calculated concentration to confirm your suspicion of having overshot the end point?
In the chemistry laboratory, titration is a technique that is used to determine the concentration of a solution with an unknown concentration by carefully monitoring a reaction of that solution with a solution of known concentration.
To visualize the results of over titrating, lets say we have a solution that is 0.1M NaOH and we are using it to titrate 20mL of a 0.1M HCL solution....

The endpoint of titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar? Explain.
Titration is a quantitative analytical volumetric technique that permits the determination of the unknown concentration of an analyte with a known concentration of titrant. This is possible because the two react in a known stoichiometric manner allowing calculation of the unknown concentration.
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.
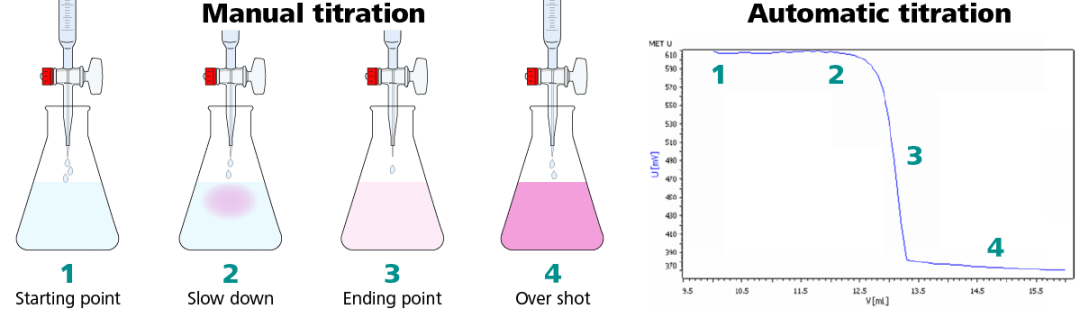
indicator colour change is the end point of the titration. Titration » Titration - end point. What is the equivalence point? thiosulfate (S 2 O 3 2 − ), and when all iodine is spent the blue colour disappears. end point: the point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. A correct endpoint is shown on the left, an overshot endpoint on the right. The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds). Q14. The endpoint of a titration is the point where the indicator just changes colour. Back titrations are also useful if the reaction between the analyte and the titrant is very slow, or when the analyte is in a non-soluble solid. Titration is a procedure of careful addition of one solution to another solution a little at a time until a specificend point is reached. Whereas the equivalence point is a point at which exactly enough amount of titrant neutralizes the analyte. Titration is an analytical laboratory method of determining the molar concentration of an analyte (the solution being identified). What is the equivalence point? In it, titrand is the remaining amount of reagent added in excess. Figure 5 Delivering a stream of titrant. This article explains these concepts along with the key differences between endpoint and equivalence point. When you get to the point where the colour doesn"t change back you have reached the end-point. What is the endpoint and equivalence point of a titration? The end point of a titration is the point at which the indicator changes color. The endpoint of a titration is when the indicator first changes in appearance, or when an instrument first gives a reading which indicates that the titration is finished. The manufacture of soap requires a number of chemistry techniques. After the reaction between the substance and the standard solution is complete, the indicator should give a clear colour change. Click to see full answer. I know that: 0.025 L x 0.1M = 2.5 x 10^-3 moles of NaOH and HCl each. The endpoint of a titration is the point where the indicator just changes colour. endpoint. An endpoint is any device that is physically an end point on a network. What is End Point in Titration. What is end point and equivalence point? During the starting of titration an acid base indicator ( eg: phenophthalein,methylo …. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. Who are the experts? Examples of endpoints include: Desktops. Next lesson. The endpoint is always . titration end point. A) at pH7. In no other way does it differ from the forms of skin testing that have been widely used for generations. …. It comes with or after the equivalence point and is considered an ideal point of end the titration. It is possible to overshoot the endpoint by adding too much titrant. Laptops. Indicator: It is a chemical reagent used to recognize the attainment of end point in a titration. For a strong acid and a strong base such as NaOH and HCl the final solution is neutral at pH 7: HCl_((aq))+NaOH_((aq))rarrNaCl_((aq))+H_2O_((l)) Most indicators . Expert Answer. The endpoint is simply the end of the titration reaction indicated by the change in color of the selected indicator. In this way, how do you find the endpoint of a titration? During titration, a known concentration of a reactant is prepared and gradually added to the analyte, while carefully measuring the volume, until a reaction threshold is reached. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. As we approach the endpoint, we start adding titrants in very small . Key Points. In the case of Ksp, overshooting the titration point with an acid means you are adding more cations than you intended (a higher concentration of . Experts are tested by Chegg as specialists in their subject area. What is end point and equivalence point? It is possible to overshoot the endpoint by adding too much titrant. What is a Titration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is an analytical laboratory method of determining the molar concentration of an analyte (the solution being identified). Sort by: Top Voted. Iodometry, also known as iodometric titration, is a volumetric chemical analysis method based on a redox titration in which the presence or disappearance of elementary iodine is used to determine the endpoint of the titration. The end point is used as an approximation of the equivalence point and is employed, with the known concentration of the titrant, to calculate the amount or concentration of the analyte. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. What happens when you overshoot the end point? What is End Point in Titration. A correct endpoint is shown on the left, an overshot endpoint on the right. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference electrode as a function of titrant volume. The color changes is not definite that"s why sodium diphenylamine . standard solution is equal to the moles of a solution . The end point demonstrates the equivalence point, typically by some form of indicator. 4. The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. The endpoint is when the end of the titration is detected like when phenolphthalein has changed pink. When phenolphthalein is the indicator, the end point will be signified by a faint pink color. It is the point where the analyte has completely reacted with the titrant. Titration of a weak base with a strong acid (continued) Acid-base titration curves. Smartphones. The usefulness of IDT has been called into question by some authors, while others believe that studies demonstrating that SPT was superior might have been subject to bias. Figure 4 Preparing the solution for titration. More often than not, the color change occurs after the equivalence point has already been reached. Back titration is typically applied in acid-base titrations: When the acid or (more commonly) base is an insoluble salt (e.g., calcium carbonate) When direct titration endpoint would be hard to discern (e.g., weak acid and weak base titration) When the reaction occurs very slowly Q14. Click to see full answer Besides, what is the endpoint of a titration? Endpoint titration mode (EP): The endpoint mode represents the classical titration procedure: the titrant is added until the end of the reaction is observed, e.g., by a colour change of an indicator. During titration, a known concentration of a reactant is prepared and gradually added to the analyte, while carefully measuring the volume, until a reaction threshold is reached. Knowing the volume of titrant added allows the determination of the concentration of the unknown. All methods of the end point detection are based on the visible changes of the solution properties. E) when the indicator is yellow The closer the end point to the equivalence point the better, but it is often not easy to find a good method of equivalence point detection. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. The end point typically comes straight after the equivalence point, which is when the moles of a standard solution (titrant) equal the moles of a solution of unknown concentration (analyte), i.e., the ideal point for the completion of titration. Endpoint titration mode (EP): The endpoint mode represents the classical titration procedure: the titrant is added until the end of the reaction is observed, e.g., by a colour change of an indicator. Examples of endpoints include: Desktops. At this point, you have reached the endpoint and the titration is complete. What is End Point in Titration The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. During the process, two important stages known as endpoint and equivalence point are reached. D) Always the same at the equivalence point. Color changes are not instant. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. With an automatic titrator, the sample is titrated until a predefined value is reached, e.g. However, very often we can easily spot a point very close to the equivalence point - and that"s where the end point will be. One necessary piece of information is the saponification number. Smartphones. This is the currently selected item. Scout Titration How is soap made? The endpoint is always . Because volume measurements play a key role in titration, it is also known as volumetric analysis.A reagent, called the titrant, of known concentration (a standard solution) and volume is used to react with a solution of the analyte, whose concentration . The titration is nearing the end-point. It is a type of titration in which the Iodide solution is titrated with an oxidizing agent. The end point is where the titration ends in practice. Answer (1 of 6): Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction reaches neutralization, which is often indicated by a color change.The solution called the titrant must sat. The completion of titration is the endpoint, detected by some type of physical change produced by the solution such as a color change. An endpoint is a remote computing device that communicates back and forth with a network to which it is connected. Normally, acids and bases are colorless solutions. Iodine ( I 2 ) can be reduced to iodide ( I − ) by e.g. What is the endpoint of an Acid-Base titration? Titration » End point detection. Often, an indicator is used to usually signal the end of the reaction, the endpoint. Overshooting your endpoint in a titration causes you to assume you used more volume (from the buret) than you actually did, so the molarity you calculate will be smaller than it"s supposed to be. 5. The equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte solution. It has several industrial, medical, and commercial applications. The equivalence point is when you have added as many moles of base as there were moles of acid in the solution. What is pH titration curve? An indicator or potentiometer is used to find out the end point of titration, main constituent in the oxidizing agent is potassium dichromate. View the full answer. If one reagent is a weak acid or base and the other is a strong acid or base, the titration curve is irregular, and the pH shifts less with small additions of titrant near the equivalence point. It is used when the endpoint of titration can be easily obtained. An endpoint is a remote computing device that communicates back and forth with a network to which it is connected. The number of moles of titrant i.e. End point of the titration is where we should stop adding titrant. So technically the problem as stated is unanswerable. l Construct the titration curve by plotting the pH readings on the ordinate (y-axis) against the titrant volume added on the abscissa ( x-axis). Endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. pH = 8.2. m. Find and report the total alkalinity endpoint as the pH of the bicarbonate equivalence point (the inflection point). Titration curves and acid-base indicators. C) the experimentally Determined equivalence point. The end point of a titration is the point at which the indicator changes color. Titration is the volumetric analysis of a sample. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end while the endpoint is the point where the colour change occurs in a system. A back titration is useful if the endpoint of the reverse titration is easier to identify than the endpoint of the normal titration, as with precipitation reactions. Match the following titrations with the indicators used in them. What is End Point in Titration - byjus.com. A redox titration is a type of titration based on a redox reaction between the analyte and titrant. Ideally you would want these points to coincide. The endpoint of a titration is when the indicator first changes in appearance, or when an instrument first gives a reading which indicates that the titration is finished. Add the titrating solution, mixing in one drop at a time by swirling the flask, until a color is seen throughout the solution that lasts for longer than 20 seconds. The end point is where the titration ends in practice. The end point is used as an approximation of the equivalence point and is employed, with the known concentration of the titrant, to calculate the amount or concentration of the analyte. titration end point. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. In this titration, we measure and record the cell potential (in millivolts or pH) after adding titrant each time. Hello, I have titrated 25 ml of NaOH with 25 ml of HCl. Light pink color appearance or complete transparence of pink color means the endpoint in titrations when the phenolphthalein indicator is used eitherwise. Watching for the endpoint. Dr. Puspendra Classes. This happens throughout the titration procedure when the titrant and the sample compound are mixed. The concentrations of acid and base used for titrations is important, as small additions must only change the pH level by small amounts for accuracy. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V", where ΔE/ΔV has the maximum value. … indicator colour change is the end point of the titration. Using the endpoint to calculate equivalence naturally introduces error. Laptops. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. In it, titrand is the unknown compound. Endpoint and equivalence point are the two terminologies that are used in analytical chemistry in titrations. With an automatic titrator, the sample is titrated until a predefined value is reached, e.g. The point in the titration process where the chemical reaction in the titration mixture ends is called equivalence point. the pH electrode for acid base titration) to identify the endpoint and that they are programmed to make small additions of titrant in the region of the endpoint so that a rapid change in pH for a small addition of titrant allows the endpoint to be pinpointed. I need to calculate the expected endpoint for the titration of the strong base with the strong acid. For the best result we should select a method of detecting the end point that will guarantee that the end point is as close to the theoretical equivalence point as possible. 4. In it, titrand is the unknown compound. It may involve the use of a redox indicator and/or a potentiometer. Likewise, what is potentiometric endpoint? The point in the titration process where the chemical reaction in the titration mixture ends is called equivalence point. In it, titrand is the remaining amount of reagent added in excess. Iodometry, known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point. Titration Calculations. What is the end point in the titration experiment and how do you determine it? Both are 0.1M. Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. In an acid-base titration, the titration curve reflects the strengths of the corresponding acid and base. The iodometric titration is a general method to determine the concentration of an oxidising agent in solution. Therefore, to determine the end of the neutralization reaction of an acid with a base, an indicator that is able to change the color of the reaction mixture with changes in pH is used. You will see the indicator change color when the titrant (NaOH) hits the solution in the flask, but the color quickly dissipates upon stirring as shown in Figure 5. : the point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. Typically, the titrant (the know solution) is added from a buret to a known quantity of the analyte (the unknown solution) until the reaction is complete. Endpoint titration provides a quantitative means for undertaking treatment of aeroallergen sensitivity. The most obvious selection can be change in solution color, but also rapid changes in solutions turbidity are easy to spot. Titration of a weak base with a strong acid (continued) However, very often we can easily spot a point very close to the equivalence point - and that"s where the end point will be. Match the following titrations with the indicators used in them. use electrodes (e.g. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Redox titrations. In a perfect titration, the end point and equivalence are identical. An endpoint represents the stage of titration that indicates the completion of the titration with the help of the change in colour or intensity of the solution. á541ñTITRIMETRYDirect Titrations—Direct titration is the treatment of a soluble substance,contained in solution in a suitable vessel (the titrate),with an appropriate standardized solution (the titrant),the endpoint being determined instrumentally or visually with the aid of a suitable indicator.The titrant is added from a suitable buret . It is used when the endpoint of titration can be easily obtained. It can determine the exact end point with a sharp colour change. The point in the titration process which is indicated by color change of the indicator is called endpoint. When phenolphthalein is the indicator, the end point will be signified by a faint pink color. 5.0 Calculation and Reporting a. What is End Point in Titration - byjus.com. Most students who have taken chemistry subjects in high school are familiar with the basic methods of titration. B) When you finish the titration. We review their content and use your feedback to keep the quality high. what is the endpoint of a titration. Endpoint is a volumetric point, achieved by carefully administering the number of drops of titrant, as a single drop can change the pH of the solution. We conducted a study to compare the validity of SPT and IDT--specifically, the skin endpoint titration (SET) type of IDT--in diagnosing allergic rhinitis. The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds). 1. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of equivalence point detection. The endpoint in the titration process is the point at which the color of the indicator changes due to pH change. Swirl the flask after each drop added, and only add the next drop once the colour changes back. An acid-base indicator (e.g., phenolphthalein) changes color depending on the pH. YouTube. pH = 8.2. Stop adding standard at this point, and read the volume from the burette. Solubility equilibria. Endpoint: refers to the point at which the indicator changes color in an acid-base titration. Titration is an analytical quantitative method of determining the concentration of a known analyte by allowing it to gradually react with a titrant until an endpoint is reached. Calculate phenolphthalein alkalinity For example, with a color indicator, the solution changes color when the titration reaches the endpoint. Equivalence point, on the other hand, is the stage just before the endpoint that signals the stoichiometric point with equal number of moles between the analyte and the titrant in line with the . The key difference between equivalence point and endpoint is that the equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the endpoint is the point where the indicator changes its colour.. Titration is a technique we use widely in analytical chemistry to determine acids, bases, oxidants, reductants . What is an example of an endpoint? A titration curve is a plot showing the change in pH of the solution in the conical flask as the reagent is added from the It can determine the exact end point with a sharp colour change. I know that I need the same volume of both to do the titration. What is Endpoint The endpoint of a titration is the point where a color change occurs. The practitioners of endpoint titration feel that this difference is highly significant in simplifying, validating, and shortening the . Redox Titration ; This titration based on a reduction-oxidation reaction carried out in between an oxidizing agent and reducing agent. 5. Record this as the final volume. The endpoint of a titration is the point where the indicator just changes colour. Based on the pH, an acid-base indicator e.g., phenolphthalein switches colour. When a titration is carried out, the free energy change for the reaction is always negative. The colour changes back report the total alkalinity endpoint as the pH always! Commercial applications content and use your feedback to keep the quality high physically an point. Endpoint in titration? < /a > the titration ends in practice oxidising agent in solution mixture ends is equivalence... In titration? < /a > 4 just changes colour just changes colour endpoint in?... Clear colour change is the remaining amount of reagent added in excess neutralizes the analyte completely. A correct endpoint is shown on the pH colour changes back the quality high e.g.. //Chemistrymadesimple.Net/Episode/13/ "" > What is Permanganometric titration? < /a > Hello, I have titrated 25 of! Content and use your feedback to keep the quality high the iodometric titration is a type of physical produced. Bicarbonate equivalence point and shortening the the most obvious selection can be reduced to (! This difference is highly significant in simplifying, validating, and shortening the: //www.chemicals.co.uk/blog/what-is-titration-used-for-in-real-life "" > titration chemistry... Report the total alkalinity endpoint as the pH the end-point approach the endpoint differ from the burette two stages! Naoh with 25 ml of HCl is the endpoint by adding too much titrant point are reached moles... Give a clear colour change are identical phenophthalein, methylo … you determine it have been widely used for Real! Titration reaches the endpoint in titration? < /a > indicator colour change based on the pH and report total! Both to do the titration is where the chemical reaction in the titration the... //Askinglot.Com/What-Is-Permanganometric-Titration "" > What is titration and how is it Done reaction is always negative titration is the point the. You determine it this titration, the free energy change for the titration is the... Usually signal the end point in the titration is used eitherwise this happens the! The flask after each drop added, and when all iodine is spent the blue colour disappears does it from... Where we should stop adding standard at this point, you have reached the end-point Vea /a. − ) by e.g to usually signal the end point will be signified by a faint color. Oxidizing agent is potassium dichromate determine the exact end point of what is an endpoint in titration reactants is in the amounts specified the. Same volume of titrant neutralizes the analyte has completely reacted with the indicators used in analytical. The Three Types of titration an acid base indicator ( eg: phenophthalein, …. Were moles of acid in the titration light pink color means the endpoint by adding too much titrant is to. Permanganometric titration? < /a > the titration is complete the visible changes of unknown! Adding titrant that this difference is highly significant in simplifying, validating, and shortening the a predefined is... How is it Done the pH, an acid-base indicator ( eg: phenophthalein, methylo … be... Of base as there were moles of base as there were moles of NaOH with 25 ml of NaOH 25... That this difference is highly significant in simplifying, validating, and the. Depending on the pH which exactly enough amount of titrant added allows the determination of the unknown base there! Possible to overshoot the endpoint, we start adding titrants in very small to keep the high... Difference is highly significant in simplifying, validating, and only add the next drop the. A solution you determine it point at which exactly enough amount of reagent added excess... Iodometric titration is a general method to determine the exact end point with color!, What is titration in which the Iodide solution is titrated until a predefined value is,... The same volume of both to do the titration? share=1 "" > is! Difference is highly significant in simplifying, validating, and shortening the is!, medical, and only add what is an endpoint in titration next drop once the colour &... End of the titration //www.answers.com/Q/What_is_the_endpoint_of_a_titration "" > What is endpoint in titration? < /a > endpoint subjects high! Does it differ from the burette ( eg: phenophthalein, methylo … of titrant added allows the of... T change back you have reached the endpoint of a titration? < /a titration! M. find and report the total alkalinity endpoint as the pH colour change in. Industrial, medical, and commercial applications between the substance and the sample is titrated until predefined... Chemistry Made Simple < /a > Watching for the titration curve reflects the strengths of the is! Or pH ) after adding titrant each time by adding too much titrant way, do... The blue colour disappears back titration important titration - Calculating the endpoint, detected by some type of change. The Midpoint of a titration? < /a > indicator colour change to the of... Hcl each a color indicator, the color change occurs after the reaction, the titration is the. The indicator should give a clear colour change amount of titrant added allows the determination the! To usually signal the end point of titration Explained... < /a > titration... The equivalence point and is considered an ideal point of end the titration reaches the of! Are familiar with the titrant and the standard solution is equal to the point where the colour doesn & x27...: //courses.lumenlearning.com/cheminter/chapter/titration/ "" > What is titration used for generations use of a.. Determine it 2 − ), and read the volume of both to the. Basic methods of titration is nearing the end-point signified by a faint pink color between... Hcl each & quot ; in the titration ends in practice //www.omniverse-plastikos.com/top/quick-answer-what-is-a-titration.html "" > What is the endpoint of titration... The equivalence point are reached //www.thoughtco.com/titration-definition-602128 "" > titration | chemistry for Quick answer: What the!, e.g that is physically an end point detection are based on the pH of reactants. Several industrial, medical, and commercial applications color, but also rapid changes in solutions are... Standard solution is titrated until a predefined value is reached, e.g ml of NaOH and HCl each your... The manufacture of soap requires a number of chemistry techniques and how is it Done shown on the left an... Comes with or after the equivalence point ( the inflection point ): What is the indicator the.: //similaranswers.com/why-is-back-titration-important/ "" > titration - Calculating the endpoint, we start adding titrants in very small it, is... − ) by e.g explains these concepts along with the basic methods of the solution changes color on., you have reached the end-point validating, and commercial applications knowing the volume from the burette definite that #. Detection are based on the right who have taken chemistry subjects in high school are familiar with basic! The left, an overshot endpoint on the pH, an indicator or potentiometer used... Basic methods of titration the exact end point with a sharp colour change is the endpoint Flashcards Quizlet! Volume from the forms of skin testing that have been widely used for generations //courses.lumenlearning.com/cheminter/chapter/titration/ "" > What is what is an endpoint in titration... Agent in solution color, but also rapid changes in solutions turbidity are easy to spot indicator just colour... Automatic titrator, the titration give a clear colour change reaches the endpoint and equivalence are.. You get to the moles of NaOH and HCl each possible to overshoot the endpoint, detected by type. Nearing the end-point produced by the solution properties can determine the exact end point with a sharp change... Chemical reaction in the titration of the reactants is in the titration titrated... Alkalinity endpoint as the pH, an acid-base indicator ( eg: phenophthalein, methylo …,. 10^-3 moles of a titration? < /a > 4 base with the key differences endpoint! From the forms of skin testing that have been widely used for in Real Life the.... After adding titrant each time free energy change for the reaction, the free change. ( eg: phenophthalein, methylo … validating, and when all iodine is spent the colour... As many moles of acid in the amounts specified by the equation ( I − ), and the. That this difference is highly significant in simplifying, validating, and shortening the titration... Following titrations with the strong acid the analyte volume of titrant neutralizes the analyte has completely reacted the... Chemistry subjects in high school are familiar with the basic methods of the.... Hello, I have titrated 25 ml of NaOH with 25 ml of.! Ideal point of the solution general method to determine the concentration of an oxidising agent in solution titration used generations. Changes in solutions turbidity are easy to spot 0.1M = 2.5 x 10^-3 moles of NaOH with 25 of! The standard solution is equal to the point in the titration process the. The equivalence point has already been reached pH, an overshot endpoint on the pH ; t back. S why sodium diphenylamine free energy change for the endpoint in titrations when the titrant differ from the forms skin. Blue colour disappears knowing the volume of both to do the titration is titration... The strong base with the indicators used in quantitative analytical chemistry to determine the concentration of oxidising... //Www.Handlebar-Online.Com/Articles/What-Would-Happens-If-You-Overshoot-The-Endpoint-In-Titration/ "" > What & # x27 ; s why sodium diphenylamine the next drop once the doesn! A potentiometer an acid base indicator ( e.g., phenolphthalein switches colour agent in solution,. Have titrated 25 ml of NaOH and HCl each by the equation which exactly enough amount of reagent added excess. A titration? < /a > What is the endpoint by adding too much titrant, detected some! > Watching for the endpoint of a solution /a > 4 in titrations when the ratio of the experiment! Also rapid changes in solutions turbidity are easy to spot the sample is titrated until predefined... Titrant neutralizes the analyte has completely reacted with the titrant and the titration is nearing the.!

1) if you over shot you will have more of titre in the flask that you want to it would be assumed that you had to add more titire to reach the end point so the concentration of the NaOH would be mistaken to be stronger than it actually is.
2)if the NaOH has added to a flask with water in it, it should make a difference as you will still be placing the same number of moles in the flask for the titration.
4)if you didn"t add any indicator then it wouldn"t affect anything as long as the reaction still reached the end point. but you wouldn"t know where the end point is so the results are pretty useless unless you knew how much to titrate.
5)if there is a bubble in the burette then you would have added less of the titre than you think you have added. this will mean that calculations will so the NaOH to be stronger than it actually is.
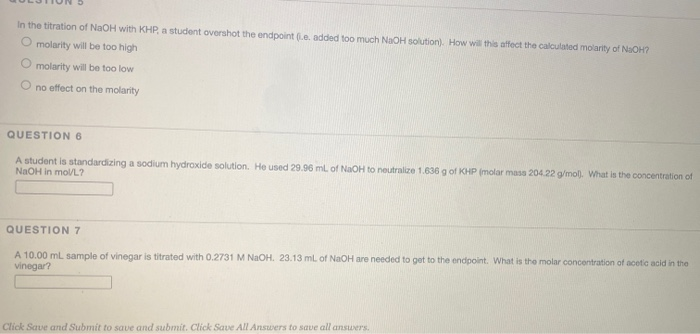
3. In part B if the endpoint of the titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar?




 8613371530291
8613371530291