the endpoint of the titration is overshot free sample

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

The endpoint of titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar? Explain.
Titration is a quantitative analytical volumetric technique that permits the determination of the unknown concentration of an analyte with a known concentration of titrant. This is possible because the two react in a known stoichiometric manner allowing calculation of the unknown concentration.
Hello guys. So in this question we have been given The sample of any work that is 1.0.10 011032 Molar. And we have been given the volume Which is equal to 25. So the sample of and which added was 28.06 Mulan 28.6 ml. And it took 3.47 ml off 0.0 0.1094 Kolarov. So we have to calculate the molar concentration of original vinegar solution, Mueller concentration. Mhm. An eagle solution. So so this is the question of backed iteration. So first we have to calculate molds of SCL added to neutralize exist and a poet. So molds of steel Is equal to 0.10 94 Mueller multiplied by 3.47 ml. So we have done more clarity multiplied by volume. So here it will be equal to 0.379612 mm. Noel. So we have founder moral status 0.379612 million malls and now they are an ascetic acid, voter mono protic acid and analyzes also mono basic and factor All assets and bases will be equal to one so minimal of base reacted with SCL which is equal to $0.379612 million total. The liberals will be equal do of based Will be zero 1032 multiplied by 28.06 Minimal which will be equal to 2.9 0.89 five 792 believe board. So now now this is total minimal of peace and now many molds of base used for vinegar will be. What is this? 2.895 792 -0.3 79612 mins. Which will give us a value of this is based used or vinegar which will be equal, do 2.51618 Milliman. And now many molds of base used for aspect asserts will be equal to medieval and acetic acid, which is more clarity multiplied by volume and it is 2.5 16 18 minimal, which is equal to 25 ML, which is volume multiplied by polarity. And from here we will they"re done modularity. So RM will be equal to 0.100 64 72 molars to the system modularity of original vinegar. I hope you understood the question. Thank you.

3. In part B if the endpoint of the titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar?

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

Sketch out a plot representing the titration of a strong monoprotic acid by a strong base, or of a strong base titrated by a strong acid. Identify the equivalence point and explain its significance.
Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Identify the equivalence point and half-equivalence points.
When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. Explain the most common reasons for this.
The objective of an acid-base titration is to determine \(C_a\), the nominal concentration of acid in the solution. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction
in which \(n\) is the number of replaceable hydrogens in the acid. The point at which this reaction is just complete is known as the equivalence point. This is to be distinguished from the end point, which is the value we observe experimentally. A replaceable hydrogen atom (sometimes called an "acidic" hydrogen) is one that can be donated to a strong base — that is, to an OH– ion. Thus in acetic acid HCOOH, only the hydrogen in the carboxyl group is considered "replaceable".
What we actually measure, of course, is the volume of titrant delivered by the burette. Learning to properly control the stopcock at the bottom of the burette usually requires some instruction and practice, as does the reading of the volume. For highly precise work, the concentration of the titrant itself must be determined in a separate experiment known as "standardization".
A plot showing the pH of the solution as a function of the quantity of base added is known as a titration curve. These plots can be constructed by plotting the pH as a function of either the volume of base added, or the equivalent fraction \(ƒ\) which is simply the number of moles of base added per mole of acid present in the solution. In most of the titration curves illustrated in this section, we plot pH as a function of \(ƒ\). It"s worth taking some time to thoroughly familiarize yourself with the general form of a titration curve such as the one shown below, in which a weak acid HA is titrated with a strong base, typically sodium hydroxide.
is stoichiometrically complete; a solution initially containing n moles of a monoprotic acid HA will now be identical to one containing the same number of moles of the conjugate base A–. At the half-equivalence point ƒ = 0.5, the concentrations of the conjugate species are identical: [HA] = [A–]. This, of course corresponds to a buffer solution (hence the relatively flat part of the curve) whose pH is the same as pKa.
As base is added beyond ƒ = 1, the pH begins to level off, suggesting that another buffered system has come into play. In this case it involves the solvent (water) and hydroxide ion: {H2O} ≈ {OH-}.
A similar effect is seen at the low-pH side of the curve when a strong acid is titrated, as in the plot for the titration of HCl below. In this case, the buffering is due to {H3O+) ≈ {H2O}.
How can this be? Surely, the concentration of OH–, even when the pH approaches 14, cannot be anything like that of [H2O] which will be about 55.5 M in most solutions! This fine point (along with the mention of H2O/OH– buffering) is rarely mentioned in elementary courses because the theory behind it involves some rather esoteric elements of solution thermodynamics. However, in case you are curious, note that the curly brackets in {H2O} ≈ {OH–} denote activities, not concentrations. And by convention the activity of a pure liquid (H2O in this case) is unity. At a pH of around 12, pOH = 2, [OH–] = .01. At this rather high ion concentration, {OH–} will be somewhat smaller than this, but the two activities will be similar enough to produce the buffering effect we observe.
The pH of the solution at its equivalence point will be 7 if we are titrating a strong acid with strong base, as in HCl + NaOH → H2O + NaCl. However, if the acid is weak, as in the above plot, the solution will be alkaline. This pH can be calculated from Cb and Kb in a manner exactly analogous to that used for calculating the pH of a solution of a weak acid in water.
It is important to understand that the equivalent fraction ƒ of base that must be added to reach the equivalence point is independent of the strength of the acid and of its concentration in the solution. The whole utility of titration as a means of quantitative analysis rests on this independence; we are in all cases measuring only the total number of moles of “acidic” hydrogens in the sample undergoing titration.
Although the strength of an acid has no effect on the location of the equivalence point, it does affect the shape of the titration curve and can be estimated on a plot of the curve.
The weaker the acid being titrated, the higher the initial pH (at ƒ=0), and the smaller will be the vertical height of the plot near the equivalence point. As we shall see later, this can make it difficult to locate the equivalence point if the acid is extremely weak.
it will be apparent that this equation reduces to pH = pKa when the titration is half complete (that is, when [HA] = [A–]), the pH of the solution will be identical to the pKa of the acid. This equation does not work for strong acids owing to the strong buffering that occurs at the very low pH at which ƒ = 0.5.
As indicated here, the buffering has nothing to do with the acid HCl itself (which does not exist as such in water), but rather with its dissociation products H3O+ and OH–, "the strongest acid and base that can exist in water."
It is important to understand the reasons for these two relations. The second is the simplest to explain. Titration of an acid HA with a base such as NaOH results in a solution of NaA; that is, a solution of the conjugate base A–. Being a base, it will react with water to yield an excess of hydroxide ions, leaving a slightly alkaline solution. Titration of a weak base with an acid will have the opposite effect.
The extent of the jump in the pH at the equivalence point is determined by a combination of factors. In the case of a weak acid, for example, the initial pH is likely to be higher, so the titration curve starts higher. Further, the weaker the acid, the stronger will be its conjugate base, so the higher will be the pH at the equivalence point. These two factors raise the bottom part of the titration curve. The upper extent of the curve is of course limited by the concentration and strength of the titrant.
These principles are clearly evident in the above plots for the titrations of acids and bases having various strengths. Notice the blue curves that represent the titration of pure water (a very weak acid) with strong acid or base.
When both the titrant and sample are "strong", we get long vertical plots at ƒ = 1. Adding even half a drop of titrant can take us across the equivalence point!
"Weak/weak" titrations tend to be problematic as the buffered regions move closer to ƒ=1. The equivalence point pH of 7 in these examples reflects the near-equality of pKa and pKb of the reactants.
It can be difficult to reliably detect the equivalence point in the titration of boric acid (pKa = 9.3) or of other similarly weak acids from the shape of the titration curve*. *An interesting student laboratory experiment that employs an auxiliary reagent (mannitol) to make boric acid stronger and thus more readily titratable was described in J. Chem Ed. 2012, 89, 767-770.
The problem here is that aqueous solutions are buffered against pH change at very low and very high pH ranges. An extreme example occurs in the titration of pure water with a strong acid or base. At these extremes of pH the concentrations of H3O+ and of OH– are sufficiently great that a competing buffer system (either H3O+/H2O or H2O/OH–, depending on whether the solution is highly acidic or highly alkaline) comes into play.
The above plots clearly show that the most easily-detectable equivalence points occur when an acid with is titrated with a strong base such as sodium hydroxide (or a base is titrated with a strong acid.)
In practice, many of the titrations carried out in research, industry, and clinical practice involve mixtures of more than one acid. Examples include natural waters, physiological fluids, fruit juices, wine making, brewing, and industrial effluents. For titrating these kinds of samples, the use of anything other than a strong titrant presents the possibility that the titrant may be weaker than one or more of the "stronger" components in the sample, in which case it would be incapable of titrating these components to completion.
In terms of proton-free energies, the proton source (the acidic titrant) would be unable to deliver an equivalent quantity of protons to the (stronger) component of the mixture.
There will be as many equivalence points as there are replaceable hydrogens in an acid. Thus in the extremely important carbonate system, equivalence points are seen at both ƒ=1 and ƒ=2:
The effect of the first point is seen by comparing the titration curves of two diprotic acids, sulfurous and succinic. The appearance of only one equivalence point in the latter is a consequence of the closeness of the first and second acid dissociation constants. The pKa"s of sulfurous acid (below, left) are sufficiently far apart that its titration curve can be regarded as the superposition of those for two independent monoprotic acids having the corresponding Ka"s. This reflects the fact that the two acidic –OH groups are connected to the same central atom, so that the local negative charge that remains when HSO3– is formed acts to suppress the second dissociation step.
In succinic acid, the two –COOH groups are physically more separated and thus tend to dissociate independently*. Inspection of the species distribution curves for succinic acid (above, right) reveals that the fraction of the ampholyte HA can never exceed 75 percent. That is, there is no pH at which the reaction H2A → HA– + H+ can be said to be “complete” while at the same time the second step HA– → A2– + H+ has occurred to only a negligible extent. Thus the rise in the pH that would normally be expected as HA is produced will be prevented by consumption of OH– in the second step which will be well underway at that point; only when all steps are completed and hydroxide ion is no longer being consumed will the pH rise.
Two other examples of polyprotic acids whose titration curves do not reveal all of the equivalence points are sulfuric and phosphoric acids. Owing to the leveling effect, the apparent Ka1 of H2SO4 is so close to Ka2 = 0.01 that the effect is the same as in succinic acid, so only the second equivalence point is detected.
Whether or not the equivalence point is revealed by a distinct "break" in the titration curve, it will correspond to a unique hydrogen ion concentration which can be calculated in advance. There are many ways of determining the equivalence point of an acid-base titration.
The traditional method of detecting the equivalence point has been to employ an indicator dye, which is a second acid-base system in which the protonated and deprotonated forms differ in color, and whose pKa is close to the pH expected at the equivalence point. If the acid being titrated is not a strong one, it is important to keep the indicator concentration as low as possible in order to prevent its own consumption of OH– from distorting the titration curve.
The observed color change of an indicator does not take place sharply, but occurs over a range of about 1.5 to 2 pH units. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. Some plants contain coloring agents that can act as natural pH indicators. These include cabbage (shown), beets, and hydrangea flowers.
For a strong acid - strong base titration, almost any indicator can be used, although phenolphthalein is most commonly employed. For titrations involving weak acids or bases, as in the acid titration of sodium carbonate solution shown here, the indicator should have a pK close to that of the substance being titrated.
When titrating a polyprotic acid or base, multiple indicators are required if more than one equivalence point is to be seen. The pKas of phenolphthalein and methyl orange are 9.3 and 3.7, respectively.
The pH meter detects the voltage produced when the H+ ions in the solution displace Na+ ions from a thin glass membrane that is dipped into the solution.
A more modern way of finding an equivalence point is to follow the titration by means of a pH meter. Because it involves measuring the electrical potential difference between two electrodes, this method is known as potentiometry. Until around 1980, pH meters were too expensive for regular use in student laboratories, but this has changed; potentiometry is now the standard tool for determining equivalence points.
Plotting the pH after each volume increment of titrant has been added can yield a titration curve as detailed as desired, but there are better ways of locating the equivalence point. The most common of these is to take the first or second derivatives of the plot: d(pH)/dV or d2(pH)/dV2 (of course, for finite increments of pH and volume, these terms would be expressed as Δ(pH)/ΔV and Δ2(pH)/ΔV2 .)
The idealized plots shown above are unlikely to be seen in practice. When the titration is carried out manually, the titrant is added in increments, so even the simple titration curve
Monitoring the pH by means of an indicator or by potentiometry as described above are the standard ways of detecting the equivalence point of a titration. However, we have already seen that in certain cases involving polyprotic acids or bases, some of the equivalence points are obscured by their close proximity to others, or by the buffering that occurs near the extremes of the pH range. Similar problems can arise when the solution to be titrated contains several different acids, as often happens when fluids connected with industrial processes must be monitored.
Acid-base neutralization reactions HA + B → A– + BH+ are always exothermic; when protons fall from their level in the acid to that in the base, most of the free energy drop gets released as heat. If the acid and base are both strong (i.e., totally dissociated), the enthalpy of neutralization for the reaction
See this Wikipedia page for more on thermometric titrations, including many examples. Note also the video on this topic in the "Videos" section near the end of this page.
Thermometric titrations are not limited to acid-base determinations; they can also be used to follow precipitation-, complex formation-, and oxidation-reduction reactions.
A typical thermometric titration curve consists of two branches, beginning with a steep rise in temperature as the titrant being added reacts with the analyte, liberating heat. Once the equivalence point is reached, the rise quickly diminishes as heat production stops. Then, as the mixture begins to cool, the plot assumes a negative slope.
Although a rough indication of the equivalence point can be estimated by extrapolating the linear parts of the curve (blue dashed lines), the differential methods described above are generally preferred.
Acids and bases are electrolytes, meaning that their solutions conduct electric current. The conductivity of such solutions depends on the concentrations of the ions, and to a lesser extent, on the nature of the particular ions. Any chemical reaction in which there is a change in the total quantity of ions in the solution can usually be followed by monitoring the conductance. Acid-base titrations fall into this category. Consider, for example, the titration of hydrochloric acid with sodium hydroxide. This can be described by the equation
which shows that two of the four species of ions being combined disappear at the equivalence point. During the course of the titration, the conductance of the solution falls as H+ and Cl– ions are consumed. At the equivalence point the conductance passes through a minimum, and then rises as continued addition of titrant adds more Na+ and OH– ions to the solution.
Each kind of ion makes its own contribution to the solution conductivity. If we could observe the contribution of each ion separately, see that the slopes for H+ and OH– are much greater. This reflects the much greater conductivities of these ions owing to their uniquely rapid movement through the solution by hopping across water molecules.
However, because the conductances of individual ions cannot be observed directly, conductance measurements always register the total conductances of all ions in the solution. The change in conductance that is actually observed during the titration of HCl by sodium hydroxide is the sum of the ionic conductances shown above.
For most ordinary acid-base titrations, conductimetry rarely offers any special advantage over regular volumetric analysis using indicators or potentiometry. This is especially true if the acid being titrated is weak; if the pKa is much below 2, the rising salt line (Na+ when titrating with NaOH) will overwhelm the fall in the contribution the small amount of H+ makes to the conductance, thus preventing any minimum in the total conductance curve from being seen.
These examples illustrate two unique capabilities of conductimetric titrations: (left) Titration of a mixture of two acids and (right) Titration of a strong polyprotic acid →
In four years of college lab sessions, many Chemistry majors will likely carry out fewer than a dozen titrations. However, in the real world, time is money, and long gone are the days when technicians were employed full time just to titrate multiple samples in such enterprises as breweries, food processing (such as blending of canned orange juice), clinical labs, and biochemical research.
A titration is carried out by adding a sufficient volume \Vo of the titrant solution to a known volume \Vt of the solution being titrated. This addition continues until the end point is reached. The end point is our experimental approximation of the equivalence point at which the acid-base reaction is stoichiometrically complete (ƒ = 1). The quantity we actually measure at the end point is the volume V_ep of titrant delivered to the solution undergoing titration.
The solution being titrated is often referred to as the analyte (the substance being "analyzed") or, less commonly, as the titrand. We shall employ the latter term in what follows.
Because we are measuring the volume of titrant rather than the number of moles, we need to use its concentration to link the two quantities. So if we are titrating the base B with acid HA, the end point is reached when a volume V of HA has been added. The number of moles of HA we have added at the end point is given by the product of its volume and concentration
Equation \ref{3-3} is important!In any titration, both the volume and the concentration of the titrant are known, so the unknown concentration is easily calculated.
In titrations carried out in the laboratory, the titrant is delivered by a burette that is usually calibrated in milliliters, so it is more convenient to express MHA in millimoles and CHA in millimoles/mL (mMol ml–1); note that the latter is numerically the same as moles/L.
50.0 mL of 0.100 M hydrochloric acid is titrated with 0.200 M sodium hydroxide. What volume of NaOH solution will have been added at the equivalence point?
This page titled 13.5: Acid/Base Titration is shared under a CC BY 3.0 license and was authored, remixed, and/or curated by Stephen Lower via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.
Not sure what titration is or what you can do with it? Then you are in the right place! In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed and some tips to get better results.
Titration is a method commonly used in chemistry to figure out the amount of a chemical in a solution. This chemical is called the titrand. To figure out the amount of titrand in the solution, a known amount of a different chemical is added to the titrand"s solution; this chemical— called the titrant, or titrating solution—reacts with the titrand. By measuring how much of the titrating solution is needed to react with all of the titrand in the solution, you can calculate how much titrand was in the solution. Simply put:
Titrant (titrating solution): A chemical you add, in a known quantity, to react with the titrand and to help you calculate the quantity of the titrand in your sample.
The point at which all of the titrand has reacted is called the endpoint, or equivalence point. How do you know when the endpoint has been reached? At the endpoint, there is usually a color change in the titrand"s solution. This is caused by an indicator, which is included in the titrand"s solution just so you can see when you have reached the endpoint. The unknown amount of titrand in the titrand"s solution can usually be determined by setting up a proportion with the known amount of titrating solution that was added. How this is done depends on the exact titrating reaction that is being used.
You can watch the video below, made by the Massachusetts Institute of Technology (MIT)"s Department of Chemistry, to see titration in action. Note: This video uses an indicator that turns light pink at the endpoint, but different indicators turn different colors at their endpoints. The next section contains more information about indicators.
There are many different types of indicators used in titration experiments. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and the titrating solution. This titration tutorial will cover two commonly used indicators—starch and phenolphthalein—along with their associated reactions.
Starch is an indicator that reacts with iodine. When there is iodine present, starch reacts with it to create a blue chemical complex. This means the solution turns blue! How is this used in titration? Iodine is included in the titrating solution, and as it is added to the titrand"s solution (which includes the titrand and starch), the titrand reacts with the iodine to turn it into iodide ions (which do not react with the starch). However, as soon as all of the titrand has reacted with the iodine and the endpoint is reached, the addition of any more iodine will finally react with the starch and turn the titrand"s solution blue!
An example of titration using a starch indicator is the titration of vitamin C, which is technically ascorbic acid. Ascorbic acid reacts with iodine to make dehydroascorbic acid and iodide ions. (This reaction is technically an oxidation-reduction reaction, also called a redox reaction for short.) When ascorbic acid and starch are both in a solution, iodine will react with the ascorbic acid. So when titrating ascorbic acid, a titrating solution containing iodine is added to the titrand"s solution, which contains starch (the indicator) and ascorbic acid (the titrand), and when all of the ascorbic acid has reacted with the iodine, any more iodine added will react with the starch and turn the titrand"s solution blue! Figure 1, below, shows a picture of the endpoint of an ascorbic acid titration using starch and iodine. Because there is a known concentration of iodine in the titrating solution, by keeping track of how much solution is added, you can determine how much titrand there was.
Figure 1. The titrand"s solution turns blue-black when the endpoint has been reached in a titration using starch as an indicator (to react with iodine).
Phenolphthalein is an indicator that changes color depending on the pH of the solution it is in. The pH of a solution is a measure of how acidic or basic it is. (For a refresher, see the Science Buddies resource on
Acids, Bases, & the pH Scale.) Specifically, phenolphthalein is colorless when the pH of a solution is acidic or neutral, but when the solution becomes slightly basic, phenolphthalein turns slightly pinkish, and then darker pink as the solution becomes more basic. How is this used in titration? A base is included in the titrating solution, and it is added to the titrand"s solution, which contains an acidic titrand and phenolphthalein. As more base is added to the titrand"s solution, the pH changes, becoming more basic, and the solution changes color. Usually, with this indicator, when the titrand"s solution just starts to turn pink, you have reached the endpoint.
An example of titration usng phenolphthalein is the titration of vinegar, which is technically acetic acid. When titrating acetic acid, a titrating solution containing a base—normally sodium hydroxide—is added to the titrand"s solution, which contains phenolphthalein (the indicator) and acetic acid (the acidic titrand). (The acetic acid reacts with the sodium hydroxide in an acid-base reaction.) When the titrand"s solution becomes basic enough due to the addition of the basic titrating solution, the phenolphthalein turns the titrand"s solution slightly pink. Phenolphthalein is specifically colorless at a neutral or acidic pH, and becomes light pink as the pH becomes more basic (first turning slightly pink around a pH of 8.3). Figure 2, below, shows a picture of the endpoint of an acetic acid titration using phenolphthalein and sodium hydroxide. Because the number of moles of sodium hydroxide used to titrate the acetic acid equals the number of moles of acetic acid in the titrand solution, by keeping track of how much titrating solution is added, you can determine how much titrand there was.
Figure 2. The titrand"s solution turns slightly pink when the endpoint has been reached in a titration using phenolphthalein as an indicator (to show the change in pH).
There are many steps that should be taken to ensure that a titration is successful and that the results produced are accurate. Check out the video of best practices in titration. Here are some key points to follow and keep in mind when doing a titration:
Dissolving the starch. If you are using starch as an indicator, it is important to make sure that the starch dissolves well when you are preparing the starch solution. It may take about 15 minutes or more of stirring the starch (and crushing large pieces) in near-boiling water to dissolve the starch. If the starch does not completely dissolve, it can lead to inaccurate results.
Assembling the titration setup. Figure 3, below, shows what the general titration setup should look like. The buret is held in place by the buret clamp, which is attached to the ring stand. The titrand"s solution should be placed directly under the bottom of the buret, as shown in Figure 4, below. The buret, which can be moved up and down, should be adjusted so that it is just above the opening of the flask containing the titrand"s solution, as shown in Figure 4.
Figure 3. This picture shows a general titration setup. Note that the buret clamp is firmly attached to the ring stand. The buret shown here slides into place between the prongs of the buret clamp. The buret is held firmly in place, but can be moved up and down if needed.
Figure 4. The titrand"s solution is placed in an Erlenmeyer flask and set right below the bottom of the buret. An Erlenmeyer flask is used because its shape allows a person to swirl the solution to mix it without spilling.
Filling the buret. Before filling the buret with the titrating solution, make sure that the buret is closed at the bottom. Many burets are closed when their stopper is in the horizontal position, as shown with the red stopper in Figure 5, below. Other burets may close in different ways. Using a funnel, as shown in Figure 6, below, slowly pour the titrating solution into the top of the buret. Fill it somewhere between half full and the top mark. The exact position is not important, as long as the fluid level is not past the markings on the top of the buret. Then make sure there are no air bubbles in the funnel. To do this, put an extra beaker or flask below the buret and let a little bit of titrating solution flow into the container (or just let enough solution flow so that the entire tip of the buret is full of solution).
Reading the buret. Read the titrating solution level from the bottom of the meniscus, which is the curved surface of liquid. For example, in Figure 7, below, the level should be recorded as 21.85 milliliters (mL), since this is where the bottom of the meniscus is. Be sure to have your eyes level with the liquid level when you are reading it.
Figure 7. When reading the level of liquid in the buret, read from the bottom of the meniscus, which is being pointed to with a black arrow in this picture. For example, the level of the liquid in this buret should be read as 21.85 mL. (Note: The long white line at the top of the buret is the mark for 21 mL.)
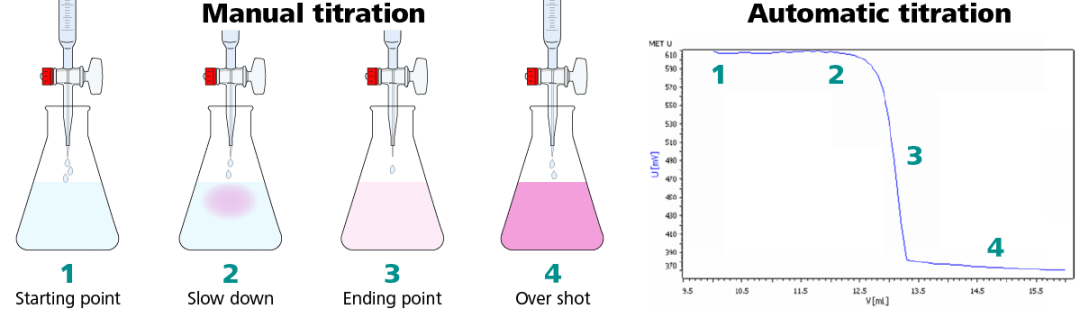
Adding the titrating solution to the titrand"s solution. Using the red stopper at the bottom of the buret, slowly add the titrating solution to the titrand"s solution one drop at a time. It is important to only let the titrating solution be added one drop at a time because the titration reaction can be very sensitive. One drop may be enough to drive the reaction to completion (if it was near completion before). If more than one drop is added at a time, the data may not be as accurate as it could be. After each drop is added, swirl the flask to mix in the titrating solution. When adding the titrating solution, you may see a temporary color change that goes away when you swirl the flask, as shown in Figure 8, below. If this happens, continue adding one drop at a time; you have reached the titration endpoint when there is a more lasting color change throughout the entire titrand"s solution, as shown in
Figure 8. This example of titrating ascorbic acid using an iodine titrating solution shows how you may see a temporary (dark blue) color change when adding a drop of the iodine solution. The temporary color change goes away when the iodine solution is mixed in the flask by swirling it.
Watching for the endpoint. Add the titrating solution, mixing in one drop at a time by swirling the flask, until a color is seen throughout the solution that lasts for longer than 20 seconds. At this point, you have reached the endpoint and the titration is complete. If you are using starch as an indicator, your endpoint may look similar to Figure 1, whereas if you are using phenolphthalein as an indicator, your endpoint may look similar to Figure 2. Note that different indicators will cause the endpoint to have a different color; you should confirm the expected color of your endpoint before starting the titration. Lastly, it is important to not overshoot the endpoint (by adding too much titrating solution) because this can cause your results to be inaccurate.
Troubleshooting: No color change is seen. There are a number of reasons why a titration may not work. Here are the most common problems that can lead to a titrand"s solution not changing colors:
Using the wrong substances. Make sure that you are using Lugol"s iodine solution or another iodine solution sold as a starch indicator and not a pure potassium iodine solution. Also confirm that you are using a soluble starch.
Using incorrect concentrations. If you are performing an ascorbic acid titration and the standard solution is too concentrated, or your titrating solution is too diluted, or your indicator solution is not the correct concentration, it may require more than 50 mL of iodine solution to titrate the sample. Note: When doing an ascorbic acid titration, the most common problem here is an overly diluted iodine solution; sometimes the Lugol"s iodine solution sold in stores is already diluted and you do not need to dilute it more.
Using too much titrand. If there is a large amount of titrand in the titrand"s solution, it may require more than 50 mL of titrating solution for it to change color. You could try using a smaller amount of titrand.
The unknown amount of titrand in the titrand solution can usually be determined by setting up a proportion with the known amount of titrating solution that was added. How this is done depends on the exact titrating reaction that is being used.
For example, if you are titrating ascorbic acid using an iodine titrating solution, you need to titrate an ascorbic acid standard, which is a sample of ascorbic acid with a known amount of ascorbic acid in it. Knowing how much iodine titrating solution is needed to titrate this known amount of ascorbic acid, you can set up a proportion to determine how much ascorbic acid is in other samples that were titrated. See Equation 1, below, for how this can be done:
Equation 1 can be rearranged to directly solve for the unknown amount of ascorbic acid in the sample ("Ascorbic Acid2"). This gives us Equation 2, below:
As an example, if you were using 20 mL of a 1 mg/mL ascorbic acid standard solution, this means you had 20 mg of titrand in your standard solution ("Ascorbic Acid1"). If it took 8.5 mL of iodine titrating solution ("Iodine1") to titrate this 20 mg, but to titrate an unknown sample of ascorbic acid it took 6.8 mL of iodine titrating solution ("Iodine2"), you can use the equation to determine that the amount of ascorbic acid in the unknown sample ("Ascorbic Acid2") equals 16 mg.
In the example titration using phenolphthalein in the titration of acetic acid, the unknown amount of acetic acid (the titrand) can again be determined by setting up a proportion with the known amount of sodium hydroxide (the titrating solution). Specifically, the number of moles of sodium hydroxide used to titrate the acetic acid equals the number of moles of acetic acid in the titrand"s solution. For example, if you added 12.5 mL (0.0125 liters [L]) of a 0.1 molar (M, which is moles/L) sodium hydroxide to titrate the acetic acid, the number of moles of both sodium hydroxide and acetic acid would be 0.0125 L x 0.1 moles/L = 0.00125 moles. You could divide by the amount of the sample (in liters) to determine the molar concentration of the acetic acid. For example, if your sample volume was 1.5 mL (0.0015 L), it would have a molarity of 0.00125 moles / 0.0015 L = 0.833 M.
Tabacco, S. and Siddiqui, A. (2003). The Digital Lab Techniques Manual: Titration. Massachusetts Institute of Technology (MIT). Department of Chemistry. Retrieved November 8, 2013.
Shodor Education Foundation, Inc. (n.d.). Redox Reactions. The University of North Carolina at Chapel Hill. Department of Chemistry. Retrieved November 10, 2021.

This invention relates to the quantitative chemical analysis of liquids by means of volumetric titration, and aims to provide a device that is more compact, rugged, and easier to use than the presently available apparatus, while maintaining accuracy of measurement.
The analysis of fluids for a specific chemical constituent is often accomplished by a procedure known as titration, in which a standard solution is mixed in increments with a sample to which has been added a color-forming indicator so that a marked color change occurs at the point where the amount of standard solution just neutralizes all of the constituent present in the sample. At this endpoint, the amount of the unknown constituent in the sample may be ascertained from the amount of standard solution used.
The basic apparatus used for titrations has hardly changed since the beginning, and remains cumbersome and difficult to use. The unknown is delivered to a titration flask with a pipette, then standard is added by means of a burette until the endpoint is reached. Specifically, it suffers from the following disadvantages:
a) The apparatus is of multiple pieces. At a minimum, six pieces are required: a pipette, a burette, a burette stand, a burette clamp, a titration flask, and a funnel.
e) The insides of the pipette and burette must be kept scrupulously clean to avoid drainage errors. This may require the use of dangerous or toxic cleaning agents.
f) The burette must be rinsed before use with the standard. This takes time and wastes standard. Also, standard remaining in the burette at the end of a series of titrations must be discarded.
h) Burette measurements are made from the position of the meniscus. The meniscus is curved and is difficult to view. If viewed from an angle, a paraflax error may be made.
i) A small amount of unknown or standard may be splashed on the side of the titration flask. The titration must be paused to wash down this deposit, or a titration error will occur.
j) Any partial droplet on the tip of the burette is shown by burette reading as having been delivered, but has not been delivered to the titration flask. For best accuracy, it must be washed off into the titration flask.
k) The contents of the flask must be mixed by swirling. Therefore, two hands are required, one to control delivery from the burette, the other to swirl the flask. This may be tiring to the operator.
l) The operator must add precisely the right of amount of standard to achieve the endpoint. One must proceed cautiously or too much standard will be added, overshooting the endpoint. A good deal of time may be consumed doing a titration because of fear of overshooting the endpoint. This is especially true for an inexperienced operator. If the end point is overshot, the operator must then repeat the titration, or live with a less than optimum result.
n) The apparatus is most accurate when a substantial portion of the contents of the burette is used for a titration. Therefore, to attain the required accuracy, it may be necessary to repeat the titration using a different amount of unknown, either by using a different pipette or by quantitatively diluting the unknown; or it may be necessary to use a standard of greater or lesser strength.
o) It is sometimes advantageous or necessary to perform reverse titrations, where the standard is titrated with the unknown. It is very inconvenient to do a series of reverse titrations, as the buret must be drained and filled with each new unknown.
Improvements have been made upon the basic apparatus. The glassware may be replaced by plastic. The burette can be arranged so that it is automatically filled. The burette may be replace by a dispenser with digital readout. Stirring may be done with a magnetic stirrer and stir bar. However, the basic manipulations remain the same, with the result that performing a titration remains a complex and time consuming matter. Simpler methods using drop counting have been described and are used, however they are of limited accuracy.
Automated analyzers have been developed, however they are expensive and are best used for the analysis of many similar samples. They are not suited for field or educational use, or the analysis of a small number of samples. U.S. Pat. No. 5,817,954 issued to Kahng et. al. on Oct. 6, 1998, shows how the apparatus for automatic titration can be simplified, using some of the same ideas as the present patent.
It is the object of this invention to provide an apparatus and technique for titration that is superior to the existing apparatus. Specifically, some advantages are:
f) No standard is wasted in rinsing the apparatus before use. The apparatus may be made of such a size that lesser amounts of standard and unknown are required, compared to the standard apparatus.
h) The amounts of unknown and standard are read from a ruled scale, with or without vernier, or from a digital display. Reading of the position of a meniscus is eliminated.
l) There is very good indication of the nearness to the endpoint, and the adjustment to the endpoint is rapid and easily done. Therefore, the time required to do a titration is much reduced.
q) The apparatus is easily customized or programed so that the results of titrations of a given sort can be directly read from the scale or digital display, with no computation required.
FIG. 1 shows a section view of one embodiment of the apparatus of the invention; and FIG. 2 shows a perspective view of another embodiment of the invention.
The preferred embodiments of the invention will hereinafter be described in conjunction with the appended drawings, where like designations denote like elements, and in which: 22 syringe barrel 24 vernier scale 26 syringe plunger 28 scale 30 needle 32 magnetic stir bar 34 magnets 36 electric motor 38 battery 40 switch with speed control 42 off/on switch 44 sensor 46 meter for sensor 48 thumb wheel 50 displacement sensor 52 volume display 54 holder 56 beaker 58 microprocessor with controls and display
A syringe barrel 22 is constructed of material resistant to the chemicals used during the titration. Glass and various plastics are suitable. A needle 30 is attached to the syringe barrel 22 by a fitting, or it may be cemented in place. The needle is generally of stainless steel, but small bore plastic tubing may also be used. The inside bore of the needle should be as small in bore as possible without unduly restricting the uptake and discharge of the standard and unknown. The syringe barrel 22 may also have an opening for attachment of a sensor 44, located at the base of the barrel 22 near the syringe inlet. Placement of the sensor near the inlet is important because when placed there, it can give information about the approach of the endpoint. The sensor is most commonly a pH electrode. Sensor 42 is connected to a meter for sensor 46 which may be an integral part of the titration apparatus, A vernier scale 24 imprinted upon barrel 22 is used in conjunction with a scale 28 imprinted on the plunger 26 to read the volumes used. For less accurate work, a vernier is not necessary, and a single mark on the barrel will suffice. Because it is the proportion of unknown to standard that is of interest, the divisions of the scale need not correspond to any standard unit of volume. Rather, they are chosen for maximum readability. Division into centimeters and subdivision into millimeters is a good choice. A displacement sensor 50 connected to volume display 52 may also be used to measure the volumes of unknown and standard. These sensors are in common use, the most basic application being a calipers with digital readout.
The syringe plunger 26 is constructed of materials of suitable mechanical and chemical resistant properties. The portion of the plunger that will be in contact with the liquids must be resistant to the chemicals used. Teflon, polyethylene, and polypropylene are suitable materials. The plunger is machined to provide a leak-proof seal to syringe barrel 22, or may have a groove fitted for an o-ring or rings which provide the seal. The tightness of fit of plunger 26 in barrel 22 is sufficient as to prevent inadvertent movement of plunger 26. The plunger may also have a rough surface or rack to be used with a thumb wheel 48 to provide a means of fine movement of the plunger. Small movements of plunger 26 are necessary to get exactly to the endpoint. A magnetic stir bar 32 is located within the syringe.
The magnetic stir bar 32is spun by drive magnets 34 which are spun by a electric motor 36. Electric motor 36 is powered by a battery 38, and controlled by a switch with speed control 40 or off/on switch 42. Alternately, the stir bar is controlled by a plurality of NS switched electromagnets, a known method. The stir bar must spin at a controlled rate, suitable as to allow easy addition of standard to the endpoint, as explained below.
A microprocessor with controls and display 58 may be electrically connected to displacement sensor 50. The microprocessor may be used to record the volume information, the strength of the standard, and to calculate the strength of the unknown. A holder 54 may be used to store the apparatus between uses, to charge the battery between uses, and to hold the apparatus in a fixed relationship to the unknown or standard in a beaker 56 during the titration.
The apparatus is first rinsed with water or other suitable liquid and the syringe plunger is positioned at or near the bottom. The small amount of liquid remaining in the syringe will not interfere with the titration. The beginning position is read. The needle tip is then wiped free of any adhering liquid. The syringe is held in a generally horizontal position, and the tip of the needle placed in a sample of the unknown. If the endpoint is to be detected by means of a color change, the addition of a small amount of an indicator to either the standard or unknown is generally necessary. A volume of the unknown is drawn into the syringe. The needle is withdrawn, wiped clean of unknown, and the volume read from the scale and vernier. The stirrer is then turned on. The needle is then placed in a sample of the standard and the standard is drawn up until the endpoint is reached. The rate of stirring is such that mixing is sufficiently slow so the the nearness of the endpoint can be easily ascertained, either by a change in color in the region near the inlet. or by a change in the sensor readout, the sensor being placed near the inlet. The importance of a proper rate of mixing and how this makes it easy to rapidly adjust to the endpoint cannot be overemphasized. If the mixing rate is too rapid, there will be little notice of the approach of the endpoint. If the mixing rate is too slow, excessive time is spent waiting for mixing to become complete. The small movements necessary to get exactly to the endpoint are more easily made if a thumb wheel or other means is used. At the end of the titration, the amount of standard is read. A calculation using the amount of unknown, the amount of standard, and the strength of the standard is done to give the strength of the unknown. For the most accurate work, a correction for the amount of standard left in the syringe is made. All liquid is expelled from the syringe and the apparatus is ready for the next titration.
If a series of titrations of a a given type is planned and a standard of consistent strength is available, a scale may be selected that has a mark showing the amount of unknown to be drawn up, and that will directly read the concentration of the unknown at the end of the titration, making a calculation unnecessary. For example the apparatus can be used to determine the titratable acidity of a wine, or the grape juice or other juice from which a wine is to be made. The syringe is equipped with a mark indicating the amount of unknown to be drawn up. The titration is done with a standard base solution until the endpoint is reached. Marks on the syringe plunger show directly the titratable acidity in any desired units. Thus a series of removable scales can be used with the same plunger to perform different standardized titrations.
Thus the reader will see that the titration apparatus of the invention provides a highly compact and easy to use device with many advantages over existing apparatus.
The apparatus is suitable for almost any type of volumetric titration, with the exception of those which evolve a gas, or form a precipitate which would clog the needle. The apparatus as described titrates a liquid with a liquid. A solid can be titrated if it is dissolved and drawn up in its entirety. The apparatus could also find use in the compounding of solutions, especially those that require a titration.
The accuracy attainable is limited principally by the quality of construction and the readability of the volumes. The apparatus will be most accurate when the full volume of the syringe is used, and the amounts of unknown and standard are equal. For example, with a vernier scale and a syringe travel of 70 millimeters, the unknown and the standard could each be read to 0.1 of 35 millimeters, leading to a potential accuracy of about 0.5% for the titration. If the amount of unknown is 10% of the amount of the standard, or the reverse, the liquid drawn up in lesser amount could be read to 0.1 of 7 millimeters, leading to a potential accuracy of about 1.4%. Thus, a large range of unknowns can be analyzed with a given standard without great loss of accuracy. If a displacement sensor with digital readout is fitted, the accuracy of reading is increased, as these sensors can detect a change in position of as little as 0.01 millimeter.
While the above description contains many specificities, these should not be construed as limitations on the scope of the invention, but rather as an exemplification of one preferred embodiment thereof. Many other variations are possible. For example, the movement of the plunger could be controlled by means of a motor. The stir bar could be driven by NS switched electromagnets located outside the syringe, either fixed at the bottom of the plunger, or following the movement of the plunger. The needle could be straight, and the syringe operated in a vertical position. The scale could be on the barrel and the vernier on the plunger. The stirrer could be powered by AC current instead of a battery. Accordingly, the scope of the invention should be determined not by the embodiments illustrated, but by the appended claims and their legal equivalents.

indicator colour change is the end point of the titration. Titration » Titration - end point. What is the equivalence point? thiosulfate (S 2 O 3 2 − ), and when all iodine is spent the blue colour disappears. end point: the point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. A correct endpoint is shown on the left, an overshot endpoint on the right. The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds). Q14. The endpoint of a titration is the point where the indicator just changes colour. Back titrations are also useful if the reaction between the analyte and the titrant is very slow, or when the analyte is in a non-soluble solid. Titration is a procedure of careful addition of one solution to another solution a little at a time until a specificend point is reached. Whereas the equivalence point is a point at which exactly enough amount of titrant neutralizes the analyte. Titration is an analytical laboratory method of determining the molar concentration of an analyte (the solution being identified). What is the equivalence point? In it, titrand is the remaining amount of reagent added in excess. Figure 5 Delivering a stream of titrant. This article explains these concepts along with the key differences between endpoint and equivalence point. When you get to the point where the colour doesn"t change back you have reached the end-point. What is the endpoint and equivalence point of a titration? The end point of a titration is the point at which the indicator changes color. The endpoint of a titration is when the indicator first changes in appearance, or when an instrument first gives a reading which indicates that the titration is finished. The manufacture of soap requires a number of chemistry techniques. After the reaction between the substance and the standard solution is complete, the indicator should give a clear colour change. Click to see full answer. I know that: 0.025 L x 0.1M = 2.5 x 10^-3 moles of NaOH and HCl each. The endpoint of a titration is the point where the indicator just changes colour. endpoint. An endpoint is any device that is physically an end point on a network. What is End Point in Titration. What is end point and equivalence point? During the starting of titration an acid base indicator ( eg: phenophthalein,methylo …. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. Who are the experts? Examples of endpoints include: Desktops. Next lesson. The endpoint is always . titration end point. A) at pH7. In no other way does it differ from the forms of skin testing that have been widely used for generations. …. It comes with or after the equivalence point and is considered an ideal point of end the titration. It is possible to overshoot the endpoint by adding too much titrant. Laptops. Indicator: It is a chemical reagent used to recognize the attainment of end point in a titration. For a strong acid and a strong base such as NaOH and HCl the final solution is neutral at pH 7: HCl_((aq))+NaOH_((aq))rarrNaCl_((aq))+H_2O_((l)) Most indicators . Expert Answer. The endpoint is simply the end of the titration reaction indicated by the change in color of the selected indicator. In this way, how do you find the endpoint of a titration? During titration, a known concentration of a reactant is prepared and gradually added to the analyte, while carefully measuring the volume, until a reaction threshold is reached. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. As we approach the endpoint, we start adding titrants in very small . Key Points. In the case of Ksp, overshooting the titration point with an acid means you are adding more cations than you intended (a higher concentration of . Experts are tested by Chegg as specialists in their subject area. What is end point and equivalence point? It is possible to overshoot the endpoint by adding too much titrant. What is a Titration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is an analytical laboratory method of determining the molar concentration of an analyte (the solution being identified). Sort by: Top Voted. Iodometry, also known as iodometric titration, is a volumetric chemical analysis method based on a redox titration in which the presence or disappearance of elementary iodine is used to determine the endpoint of the titration. The end point is used as an approximation of the equivalence point and is employed, with the known concentration of the titrant, to calculate the amount or concentration of the analyte. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. What happens when you overshoot the end point? What is End Point in Titration. A correct endpoint is shown on the left, an overshot endpoint on the right. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference electrode as a function of titrant volume. The color changes is not definite that"s why sodium diphenylamine . standard solution is equal to the moles of a solution . The end point demonstrates the equivalence point, typically by some form of indicator. 4. The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. The endpoint is when the end of the titration is detected like when phenolphthalein has changed pink. When phenolphthalein is the indicator, the end point will be signified by a faint pink color. It is the point where the analyte has completely reacted with the titrant. Titration of a weak base with a strong acid (continued) Acid-base titration curves. Smartphones. The usefulness of IDT has been called into question by some authors, while others believe that studies demonstrating that SPT was superior might have been subject to bias. Figure 4 Preparing the solution for titration. More often than not, the color change occurs after the equivalence point has already been reached. Back titration is typically applied in acid-base titrations: When the acid or (more commonly) base is an insoluble salt (e.g., calcium carbonate) When direct titration endpoint would be hard to discern (e.g., weak acid and weak base titration) When the reaction occurs very slowly Q14. Click to see full answer Besides, what is the endpoint of a titration? Endpoint titration mode (EP): The endpoint mode represents the classical titration procedure: the titrant is added until the end of the reaction is observed, e.g., by a colour change of an indicator. During titration, a known concentration of a reactant is prepared and gradually added to the analyte, while carefully measuring the volume, until a reaction threshold is reached. Knowing the volume of titrant added allows the determination of the concentration of the unknown. All methods of the end point detection are based on the visible changes of the solution properties. E) when the indicator is yellow The closer the end point to the equivalence point the better, but it is often not easy to find a good method of equivalence point detection. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. The end point typically comes straight after the equivalence point, which is when the moles of a standard solution (titrant) equal the moles of a solution of unknown concentration (analyte), i.e., the ideal point for the completion of titration. Endpoint titration mode (EP): The endpoint mode represents the classical titration procedure: the titrant is added until the end of the reaction is observed, e.g., by a colour change of an indicator. Examples of endpoints include: Desktops. At this point, you have reached the endpoint and the titration is complete. What is End Point in Titration The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. During the process, two important stages known as endpoint and equivalence point are reached. D) Always the same at the equivalence point. Color changes are not instant. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. With an automatic titrator, the sample is titrated until a predefined value is reached, e.g. However, very often we can easily spot a point very close to the equivalence point - and that"s where the end point will be. One necessary piece of information is the saponification number. Smartphones. This is the currently selected item. Scout Titration How is soap made? The endpoint is always . Because volume measurements play a key role in titration, it is also known as volumetric analysis.A reagent, called the titrant, of known concentration (a standard solution) and volume is used to react with a solution of the analyte, whose concentration . The titration is nearing the end-point. It is a type of titration in which the Iodide solution is titrated with an oxidizing agent. The end point is where the titration ends in practice. Answer (1 of 6): Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction reaches neutralization, which is often indicated by a color change.The solution called the titrant must sat. The completion of titration is the endpoint, detected by some type of physical change produced by the solution such as a color change. An endpoint is a re




 8613371530291
8613371530291