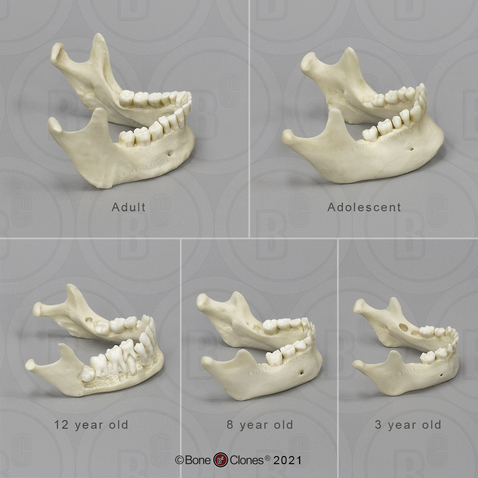
overshot jaw human for sale
Teeth will become easier to clean. Your risks for tooth decay and gum disease will decrease. You’ll also feel less strain on your teeth, jaws, and facial muscles.
Removal of one or more teeth on the lower jaw may also help improve the appearance of an underbite if overcrowding of the teeth is contributing to the issue. A dentist may also use a grinding device to shave down or smooth teeth that are large or stick out.
As the puppies grow, we see different body parts growing at different rates. Sometime, a German Shepherds puppy has a slights overbite at 8 weeks, when the teeth are not in a tight scissors bite, as they should per breed standard. As the puppy continued developing, this slight overbite usually resolves itself, as puppy gets through teething stage and has their adult teeth. Because overbite is a fault, breeders should never use dogs with any less-than-perfect teeth in breeding. (Luckily for us, humans, an orthodontic treatment exists and even those of us with the most un-perfect smiles, still able to reproduce. Dogs in a show world aren"t that lucky ). We have never seen an under-bite in this breed. While to many pet owners slight overbite might not seem like a serious condition, but a cosmetic defect, it is very important that your puppy"s teeth are aligned as close as possible. Severely misaligned teeth can lead to difficulty eating, gum injuries and bruising, bad breath and different types of dental problems, including tooth decay and gingivitis. Fortunately, there are ways to help fix the problem before it becomes irreversible.
An overbite is a genetic, hereditary condition where a dog"s lower jaw is significantly shorter than its upper jaw. This can also be called an overshot jaw, overjet, parrot mouth, class 2 malocclusion or mandibular brachynathism, but the result is the same – the dog"s teeth aren"t aligning properly. In time, the teeth can become improperly locked together as the dog bites, creating even more severe crookedness as the jaw cannot grow appropriately.
Dental examinations for puppies are the first step toward minimizing the discomfort and effects of an overbite. Puppies can begin to show signs of an overbite as early as 8-12 weeks old, and by the time a puppy is 10 months old, its jaw alignment will be permanently set and any overbite treatment will be much more challenging. This is a relatively narrow window to detect and correct overbites, but it is not impossible.
Small overbites often correct themselves as the puppy matures, and brushing the dog"s teeth regularly to prevent buildup can help keep the overbite from becoming more severe. If the dog is showing signs of an overbite, it is best to avoid any tug-of-war games that can put additional strain and stress on the jaw and could exacerbate the deformation.
If an overbite is more severe, dental intervention may be necessary to correct the misalignment. While this is not necessary for cosmetic reasons – a small overbite may look unsightly, but does not affect the dog and invasive corrective procedures would be more stressful than beneficial – in severe cases, a veterinarian may recommend intervention. There are spacers, braces and other orthodontic accessories that can be applied to a dog"s teeth to help correct an overbite. Because dogs" mouths grow more quickly than humans, these accessories may only be needed for a few weeks or months, though in extreme cases they may be necessary for up to two years.
If the dog is young enough, however, tooth extraction is generally preferred to correct an overbite. Puppies have baby teeth, and if those teeth are misaligned, removing them can loosen the jaw and provide space for it to grow properly and realign itself before the adult teeth come in. Proper extraction will not harm those adult teeth, but the puppy"s mouth will be tender after the procedure and because they will have fewer teeth for several weeks or months until their adult teeth have emerged, some dietary changes and softer foods may be necessary.

Jaw muscles are versatile entities that are able to adapt their anatomical characteristics, such as size, cross-sectional area, and fibre properties, to altered functional demands. The dynamic nature of muscle fibres allows them to change their phenotype to optimize the required contractile function while minimizing energy use. Changes in these anatomical parameters are associated with changes in neuromuscular activity as the pattern of muscle activation by the central nervous system plays an important role in the modulation of muscle properties.
This review summarizes the adaptive response of jaw muscles to various stimuli or perturbations in the orofacial system and addresses general changes in muscles as they adapt, specific adaptive changes in jaw muscles under various physiologic and pathologic conditions, and their adaptive response to non-surgical and surgical therapeutic interventions.
Although the jaw muscles are used concertedly in the masticatory system, their adaptive changes are not always uniform and vary with the nature, intensity, and duration of the stimulus. In general, stretch, increases neuromuscular activity, and resistance training result in hypertrophy, elicits increases in mitochondrial content and cross-sectional area of the fibres, and may change the fibre-type composition of the muscle towards a larger percentage of slow-type fibres. In contrast, changes in the opposite direction occur when neuromuscular activity is reduced, the muscle is immobilized in a shortened position, or paralysed. The broad range of stimuli that affect the properties of jaw muscles might help explain the large variability in the anatomical and physiological characteristics found among individuals, muscles, and muscle portions.
The jaw muscles control the position and motion of the mandible and create forces at the teeth and temporomandibular joints. The consequences of their action are pertinent to, for instance, prosthetic changes of dental occlusion, orthodontic treatment of malocclusions, and surgical craniofacial corrections. Their special functional anatomy makes jaw muscles the most complex and most powerful in the human body (Hannam and McMillan, 1994; van Eijden et al., 1997). Modifications of their contractile properties, such as contraction velocity and force generation, in response to varying functional demands, a process called ‘adaptation’, are reflected in changes of the functional anatomy of this muscle group, individual muscles, or muscle regions.
Jaw muscles, like all skeletal muscles, are capable of powerful contractions by virtue of the regular organization of their contractile proteins. The levels of skeletal muscle organization, gross to microscopic, are summarized in Figure 1. The cellular units of a skeletal muscle are the muscle fibres, long cylindrical cells with multiple nuclei. Each muscle fibre contains many myofibrils that run parallel to its length. The myofibrils are composed of sarcomeres, arranged end to end. Sarcomeres, the contractile units of a skeletal muscle fibre, consist of two types of myofilament organized into regular arrays with a partially overlapping structure. The thin filaments contain mainly actin, whereas the thick filaments contain mainly myosin. Myosin consists of two intertwined heavy peptide chains (Gazith et al., 1970) and four light peptide chains (Lowey and Risby, 1971). Because it contains the ATPase activity, which determines the contraction velocity, the myosin heavy chain (MyHC) is primarily responsible for the contraction velocity of the muscle fibre (Staron, 1991).
Human jaw muscles are different from other skeletal muscles. For instance, human jaw-closing muscles are composed of a relatively homogeneous mixture of type I and II fibres (Stålberg et al., 1986), the type II fibres being much smaller than the type I (Eriksson and Thornell, 1983; McComas, 1998; Sciote et al., 2003). This contrasts with the mosaic-like distributions of type I and II fibres in limb muscles, where type II fibre diameters are greater than type I diameters (Burke, 1981). In addition, healthy adult human jaw-closing muscles do not contain MyHC-IIB (Horton et al., 2001), but express MyHC-foetal and MyHC-cardiac alpha, MyHC isoforms typically present in developing and cardiac muscle, respectively, in addition to types I, IIA, and IIX (Sciote et al., 1994). Many of their fibres are hybrids, expressing two or more MyHC isoforms in various combinations (Monemi et al., 1996, 1998; Korfage et al., 2005a, b). These hybrid fibres have contractile properties intermediate to those of pure fibres. The diversity of MyHC isoforms and the large number of hybrid fibres present in jaw muscles under steady state conditions provides a mechanism that allows a very fine gradation of contraction velocity (Pette, 2002). Another feature that distinguishes jaw muscles from other skeletal muscles is their internal organization. While the fibres of a motor unit intermingle large parts of limb muscles, they are restricted to specific areas in the jaw muscles. Such an organization permits the differential control of separate muscle portions (van Eijden and Turkawski, 2001). Together with their complex architecture and heterogeneous fibre-type composition, this enables the jaw muscles to perform a larger variety of motor tasks than the average limb or trunk muscle.
Jaw muscles are constantly exposed to a variety of local and systemic stimuli, to which they can adjust their properties. This ability of jaw muscles to adapt to varying functional demands is obligatory for their normal function under changing environmental conditions, repair after pathology, and successful outcomes of therapeutic interventions. Although the jaw muscles are used concertedly in the masticatory system, their adaptive changes are not always uniform and vary with the nature of the stimulus.
The purpose of this article is to review the adaptive response of jaw muscles to various stimuli or perturbations in the orofacial system. It addresses (1) general changes in skeletal muscles as they adapt, (2) specific adaptive changes in jaw muscles under various physiologic and pathologic conditions, and (3) their adaptive response to non-surgical and surgical therapeutic interventions.
Notwithstanding the marked differences between human jaw muscles and other skeletal muscles, their adaptational processes follow the same general concept. While changes can occur at the level of structure, metabolism, energy storage, and function, this review will focus on neuromuscular activity, fibre cross-sectional area, and fibre-type composition.
Neuromuscular activity is an important factor for the regulation of fibre types (Hennig and Lømo, 1985; Gorassini et al., 1999). For instance, fast fibres are innervated by motoneurons that fire more rapidly and in shorter bursts than those innervating slow fibres (van Eijden and Turkawski, 2001). Furthermore, adaptive changes in the fibre-type composition of muscles are related to the duration of their activity. A positive correlation has been demonstrated between the duration of daily activation and the proportion of slow fibres in skeletal muscles (Monster et al., 1978; Kernell et al., 1998). Experiments on fibre-type transitions therefore often use protocols to either increase or decrease neuromuscular activity. Besides the duration of activity, its intensity seems to play a critical role in modulating the phenotype of skeletal muscle fibres. On the basis of observations in rabbit jaw muscles, in which the number and cross-sectional area of slow fibres were positively correlated with the activity duration only for activations exceeding 30 per cent of the maximum activity, it was concluded that activation above a specific threshold influences the fibre properties of a muscle (van Wessel et al., 2005b). Skeletal muscle fibres may thus require specific activity patterns to adjust their properties to altered environmental demands.
Although adaptational processes of jaw muscles are reflected in a broad range of anatomical and physiological parameters, studies on jaw-muscle adaptation most commonly assess muscle activity, force output, muscle size, MyHC isoform composition, and cross-sectional area of the individual fibres. Therefore, techniques to analyse these parameters are briefly described below.
The most common physiological test to determine muscle activity is electromyography (EMG), which is a recording of the amplified motor unit action potentials of the muscles (Figure 2). The EMGs of human jaw muscles are typically recorded using surface electrodes placed on the skin overlying a muscle belly (Svensson et al., 2004). However, this technique only describes general gross activity in the whole muscle and is limited to the execution of specified motor tasks (e.g. Holmgren et al., 1990; Karkazis and Kossioni, 1997, 1998). The use of intramuscular electrodes is more refined and allows the assessment of the myoelectric activity in various parts of a jaw muscle (Blanksma and van Eijden, 1995). When these electrodes are used in combination with implantable transmitters in animal experiments (Langenbach et al., 2002), it is possible to continuously record jaw-muscle EMGs over several weeks and to study changes in the myoelectric activity during a broad range of normal daily activities (Langenbach et al., 2004; Grünheid et al., 2005, 2006; van Wessel et al., 2005a).
The activation of a muscle determines its mechanical force output. Therefore, bite force transducers are often used to assess jaw-closing muscle activity as it is translated into force production at the occlusal surfaces of teeth (van Eijden, 1990). The device is placed between the maxillary and mandibular teeth, and the subjects are encouraged to clench as hard as possible (Ellis et al., 1996; Svensson et al., 1998a). Although tests of this procedure cannot discriminate between the force outputs of individual muscles, they do have a potential for studying the force of isometric contractions of the jaw-closing muscles.
The size (length, thickness, cross-sectional area, and volume) and orientation of a jaw muscle can be measured in vivo using modern imaging techniques, such as ultrasonography (e.g. Kiliaridis and Kalebo, 1991; Benington et al., 1999), computed tomography (e.g. Weijs and Hillen, 1984, 1986), and magnetic resonance imaging (e.g. van Spronsen et al., 1991; Goto et al., 2006). While ultrasonography is limited to analysing superficially located, easily accessible jaw muscles, such as the masseter, computed tomography and magnetic resonance imaging enable assessment of all jaw muscles.
The fibre types of jaw muscles can be determined immunohistochemically using monoclonal antibodies raised against various isoforms of purified MyHC (Bredman et al., 1991; Korfage and van Eijden, 2003). The cross-sectional areas of individual fibres can be quantified on microphotographs of transverse sections of the muscle. When subsequent sections are incubated with antibodies directed against various MyHC isoforms (Figure 3), the cross-sectional area of a fibre can be related to its MyHC isoform content (Korfage et al., 2000).
Example of an area showing the antero-deep portion of the human masseter incubated with antibodies against (a) myosin heavy chain (MyHC)-I, (b) MyHC-cardiac alpha, (c) MyHC-IIA, (d) MyHC-IIA and MyHC-IIX, and (e) MyHC-foetal isoforms. (f) The drawing shows some of the fibre types: (1) MyHC type I, (2) MyHC type IIX, (3) MyHC type IIA, (4) MyHC type I + IIA, (5) MyHC type foetal + I, and (6) MyHC type foetal + cardiac alpha + I.
For ethical and practical reasons, it is often impossible to assess the MyHC type composition and fibre cross-sectional area of jaw muscles in humans. Therefore, experimental studies commonly use animal models. It has to be noted, however, that results obtained from animal jaw muscles can not be uncritically extrapolated to human jaw muscles as they differ in expression of MyHC isoforms, fibre diameter, and fibre-type proportions. The muscles of large animals generally contain a higher proportion of fibres expressing MyHC-I than those of small animals (Pellegrino et al., 2003). The adaptation of jaw muscles may, therefore, differ among species and should be interpreted with due caution.
The function and properties of jaw muscles are affected by systemic and local alterations. The broad range of conditions to which jaw muscles adapt their properties provides a possible explanation for the large variability in the jaw-muscle fibres in humans (Korfage et al., 2000). In the following section, a description is given of the properties of jaw muscles, with special focus on neuromuscular activity, fibre cross-sectional area, and fibre-type composition, in relation to several physiologic and pathologic processes and conditions, such as ageing, craniofacial morphology, diet, dental status, craniomandibular disorders (CMD), and pain.
Ageing leads to systemic changes, which have a marked influence on skeletal muscles (McComas, 1998). Some of the changes are related to a reduction in the levels of anabolic hormones, such as thyroid and growth hormones (Proctor et al., 1998). Although ageing leads to reductions in muscle activity, synthesis rate for contractile proteins, contraction velocity of fibres, and muscle strength as well as to changes in the fibre-type proportions and fibre cross-sectional areas in all skeletal muscles (Larsson et al., 1997), the changes are more complex in jaw muscles than in limb and trunk muscles. In jaw muscles, ageing also coincides with a reduction in the number and amplitude of reflex responses, longer latency of oral reflexes (Smith et al., 1991; Kossioni and Karkazis, 1994), and a prolongation of muscle-contraction time (Newton et al., 1993). The pattern of relative jaw-muscle activity, as seen in EMG, does not change with age, but its amplitude is generally lower in elderly subjects (Alajbeg et al., 2006).
The thickness of the jaw muscles decreases significantly with age (Newton et al., 1987, 1993), which is caused primarily by a decrease in the cross-sectional area of their fibres (Monemi et al., 1998, 1999b). These changes might be explained by the progressive reduction in the number and the total duration of activity bursts per day with age (Miyamoto et al., 1999).
The fibre-type composition of the jaw muscles also changes with age. In the jaw-closing muscles of elderly subjects, the proportion of pure type I fibres decreases, while the proportion of pure type II fibres and that of hybrid fibres, particularly of those co-expressing MyHC-foetal, increase (Eriksson and Thornell, 1983; Monemi et al., 1996, 1999a).
Similarly, the jaw-opening muscles undergo changes in fibre-type composition during ageing. In the lateral pterygoid muscle, the proportion of fibres expressing MyHC-IIA, which are rare or absent in young adults, increases with age, whereas the proportion of fibres expressing MyHC-I decreases. These changes reflect a shift towards faster properties of this muscle in the elderly (Monemi et al., 1999b, 2000). In the digastric muscle, the change in fibre-type composition during ageing is also characterized by a decrease in the proportion of fibres expressing MyHC-IIX (Monemi et al., 1999b, 2000). This suggests a narrowing in the range of contraction velocities of the digastric muscle fibres with age. In general, however, the degenerative changes within the human masticatory motor system with age lead to muscle fibre atrophy, increased variability in fibre diameter, and a shift towards faster fibre types.
Notwithstanding the large variation in facial form among humans, three basic skeletal types are generally described: dolichofacial, mesofacial, and brachyfacial (Ricketts, 1960) with relatively long, average, and relatively short faces, respectively (Bishara and Jakobsen, 1985). The variations in craniofacial morphology have been shown to be associated with certain parameters of jaw-muscle function, including myoelectric activity (Ueda et al., 1998) and occlusal force (Ingervall and Helkimo, 1978; Proffit et al., 1983). Although the duration and the amplitude of EMG activity are similar during mandibular motion (Ueda et al., 2000; Farella et al., 2005), long face subjects generate less jaw-closing muscle activity (Bakke and Michler, 1991) and lower molar bite forces during maximum effort than subjects with normal and short faces (Ingervall and Helkimo, 1978; Proffit et al., 1983; Hunt and Cunningham, 1997).
To a limited extent, there is also a relationship between craniofacial morphology and the cross-sectional areas of the jaw muscles (van Spronsen et al., 1991). Short face subjects have thicker jaw-closing muscles than ‘normal’ subjects (Weijs and Hillen, 1984; Kiliaridis and Kalebo, 1991), whereas long face subjects have significantly thinner muscles (van Spronsen et al., 1992; Benington et al., 1999).
Moreover, the fibre-type composition of jaw-closing muscles varies with the vertical craniofacial dimension (Rowlerson et al., 2005). For instance, there is a significant negative correlation between ramus length and proportion of type I fibres in the medial pterygoid muscle (Shaughnessy et al., 1989). Typically, subjects with a short face have larger proportions of type II fibres, which are able to produce a higher maximum force of short duration, than type I fibres in their jaw-closing muscles than subjects with normal or long facial dimensions (Hunt et al., 2006).
Although it is accepted that there is a correlation between craniofacial morphology and jaw-muscle function, the question remains as to which comes first. The muscle response might be adaptive to the underlying skeletal development (Hunt et al., 2006) or it might be the driving force. Evidence from numerous animal studies suggests that jaw-muscle function can influence the shape of the skull during growth and development (for overview, see Kiliaridis, 2006). It is likely that in humans, too, the jaw muscles play a part in the control of facial form and partly determine the final facial dimensions (Weijs and Hillen, 1986).
Humans with weak jaw muscles have a greater variation in facial morphology than those with strong jaw muscles (Ingervall and Helkimo, 1978). It is believed that strong or thick muscles produce faces with similar morphologic features, whereas weak muscles cannot influence the morphology to such an extent (Kiliaridis, 1995). For instance, many of the unique morphological facial features of Inuits and Neanderthals are thought to be a response to their powerful jaw muscles and intensive mastication of a relatively hard diet (Spencer and Demes, 1993). The effects of the hardness of the available food on the structural properties of jaw muscles are discussed below.
Following obstruction of the nasal airway, the mandible is lowered to allow breathing through the mouth. The change in posture is reflected in a decrease in resting EMG activities of the temporalis muscles and an increase in those of the suprahyoid muscles (Hellsing et al., 1986). The maximum activities, however, remain unchanged (Hiyama et al., 2003). EMG changes during posture are maintained over the entire period of nasal obstruction, even over several years (Miller et al., 1984). In subjects with chronic nasal obstruction, the vertical craniofacial morphology develops towards a more dolichofacial skeletal type with increased anterior face height, overeruption of molars, and backward rotation of the mandible (Principato, 1991; Vickers, 1998). The jaw muscles adapt to their new functional lengths by increasing the number of sarcomeres in series within each fibre to re-establish the optimum overlap of the contractile proteins (Goldspink, 1998). It is conceivable that they also adjust their MyHC composition and cross-sectional area to the altered craniofacial morphology as detailed above.
The influence of mechanical food properties on human jaw muscles has been demonstrated in several studies: jaw-closing muscles alter their myoelectric activities in response to altered food hardness (Agrawal et al., 1998), the amplitudes being higher during chewing of harder food (Karkazis and Kossioni, 1997, 1998; Karkazis, 2002; Peyron et al., 2002).
Information from numerous animal experiments indicates that the consistency of daily food can also affect other physiologic features: jaw muscles of pigs (Ciochon et al., 1997) and rats (Whiteley et al., 1966) raised on a soft diet generate lower tetanic isometric muscle tension after stimulation (Kiliaridis and Shyu, 1988) than those of animals raised on a hard diet. These muscles also contain less mitochondria per unit (Sato and Konishi, 2004), providing them with poorer pre-requisites for energy production. The corresponding reduction in motor unit activity seems to be caused solely by muscular changes, but not by changes in motoneurons; studies on rats revealed no difference in the soma diameter of motoneurons between soft and hard diet groups (Miyata et al., 1993).
The jaw muscles of animals raised on diets of different hardness also differ with regard to the cross-sectional area and the MyHC composition of their fibres. Experiments on rabbits and ferrets demonstrated that the long-term intake of a soft diet, which requires less masticatory effort, caused a decrease in the cross-sectional areas of both type I and II fibres in jaw-closing muscles and of type II fibres only in jaw-opening muscles (Langenbach et al., 2003; He et al., 2004; Kitagawa et al., 2004). In rats, food hardness also affected the fibre-type composition of the jaw muscles: the intake of a soft diet resulted in more MyHC-IIB and MyHC-IIX-rich phenotype of the masseter muscle than the intake of a hard diet (Miehe et al., 1999; Saito et al., 2002).
It can thus be inferred that long-term alteration in the pattern of muscle use, caused by a reduction in the consistency of the available food, contributes to selective disuse resulting in functional and morphological adaptation of the jaw muscles, reflected in reductions in their myoelectric activity, force output, fibre cross-sectional area, and percentage of slow fibres.
A relationship exists between the activity of jaw muscles and the sagittal skeletal base relationship. In subjects with mesial jaw relationships, the postural EMG activities of the masseter and temporalis muscles are higher than in those with neutral or distal jaw relationships (Tecco et al., 2007) and are also positively correlated with the severity of the skeletal malocclusion (Miralles et al., 1991). The difference has been attributed to the muscular action axis and to increases in the gravitational component in Class III malocclusions (Cha et al., 2007). Surprisingly, during maximal voluntary contraction, EMG activities do not differ among subjects with different sagittal occlusal relationships (Miralles et al., 1991; Tecco et al., 2007). The limited evidence available suggests that the fibre-type composition does not differ either: biopsies of the masseter muscle taken from adult patients with mesial occlusions did not differ in their MyHC distribution from those of patients with distal occlusions (Gedrange et al., 2005a).
Comparison of various vertical malocclusions indicates that the EMG activities of the masseter and temporalis muscles during mastication are higher in subjects with deep bites than in those with open bites (Kayukawa, 1992). In comparison with subjects with anterior open bites, those with deep bites also have greater cross-sectional areas of the masseter muscles (Gedrange et al., 2005b). These findings are easily comprehensible as malocclusions with deep overbites and anterior open bites are often associated with brachyfacial and dolichofacial vertical craniofacial morphologies, respectively. Biopsies taken from human masseter muscles showed that the percentage occupancy of fibre types varied significantly between groups of subjects with different overbites. Greater areas of type II fibres were found in masseter muscles of deep bite subjects, whereas greater areas of type I fibres were found in masseter muscles of open bite subjects (Rowlerson et al., 2005). These findings indicate an interaction between vertical overbite and masseter muscle fibre-type composition and make it reasonable to postulate that the fibre-type composition of other jaw muscles also varies with vertical bite characteristics.
Malocclusions with transverse discrepancies also affect jaw-muscle properties: lateral displacement of the mandible affects the orientation of the jaw muscles, which in turn seems to affect their activity and size. In subjects with mandibular laterognathism, the masseter muscles are orientated more vertically and have significantly smaller lengths and volumes on the deviated side than in subjects with a normal occlusion (Goto et al., 2006). The lateral displacement of the mandible can explain the orientation differences (Figure 4) but not the smaller muscle size. It seems that the muscles on the deviated side are not only smaller but also generate less myoelectric activity during functional use. For instance, during chewing and maximum contraction, the EMG amplitudes of the masseter and temporalis muscles in patients with unilateral crossbites or lateral forced bites differ between sides, with the activity levels being lower on the crossbite side than on the normal side (Ingervall and Thilander, 1975; Ferrario et al., 1999). The volume difference is possibly the physiologic result of the different level of bilateral activity of the masticatory muscles. Treatment of the lateral displacement seems to eliminate the underlying reason for the different levels of activity as no bilateral differences in the thickness of the masseter muscles were found several years after successful correction of a unilateral crossbite (Kiliaridis et al., 2007). Lateral displacement of the mandible thus appears to initiate an adaptive process in the masticatory system, resulting in atrophy of the jaw muscles on the crossbite side. In addition to the apparent decrease in size of the muscles whose orientations are affected, which is most likely associated with a reduction in the cross-sectional area of the individual fibres, the fibre-type composition might shift towards a higher percentage of fast-type fibres.
Data on the effects of occlusal guidance on the jaw muscles are still contradictory. Although lower EMG activities of the masseter and temporalis muscles during clenching, grinding, and chewing have been reported for subjects with canine guidance than for those with group function (Shupe et al., 1984), a more recent study did not detect any differences (Borromeo et al., 1995).
More information is available on how occlusal interferences affect jaw-muscle activity. Premature contacts alter the occlusal balance and lead to smaller numbers of activity periods per hour (Michelotti et al., 2005) and to lower amplitudes of muscle activity during posture (Ingervall and Carlsson, 1982) and clenching (Borromeo et al., 1995). Lower maximum activities in the jaw-closing muscles have been demonstrated in subjects with lower numbers of occlusal contacts (Ferrario et al., 2002) and it has been suggested that reduced occlusal stability is associated with weak elevator muscle activity (Bakke and Michler, 1991). Accordingly, occlusal adjustment results in an immediate increase in myoelectric activity and contraction force of the jaw-closing muscles during maximum effort (Holmgren and Sheikholeslam, 1994). To the present, no information is available as to whether there is a relationship between the occlusal contact situation and the phenotypic properties of jaw-muscle fibres.
The loss of posterior teeth limits chewing efficiency due to a reduced occlusal area and alterations in the occlusal loads (Fontijn-Tekamp et al., 2000). While the bite forces acting on the individual residual teeth increase, the overall bite force tends to decrease with shortening of the dental arch (Hattori et al., 2003). This shortening coincides with lower activation ratios of the jaw-closing muscles as compared with those of fully dentate subjects (Hattori et al., 2003).
Tooth loss also influences anatomical features of the jaw muscles. Computed tomography analysis revealed a reduction in radiodensity, due to an increased proportion of fat tissue (Raustia et al., 1996) and cross-sectional area (Newton et al., 2004) of the masseter and medial pterygoid muscles in subjects who had been edentulous for a long period of time. Although no studies have yet been performed that compare fibre-type proportion of edentulous and dentate humans, it is likely that the muscle atrophy implied by these findings is also reflected in the MyHC composition of the jaw-muscle fibres. In rhesus monkeys, the long-term adaptive changes of the masseter and temporalis muscles following complete tooth removal include a relative increase in type IIB fibres and a relative decrease in type IIA and type I fibres (Maxwell et al., 1980). It is conceivable that the reduced ability to chew food, sensory feedback via gingival tissues in the absence of periodontal receptors, and associated reduced force production contribute to the disuse atrophy indicated by the change in fibre-type composition.
The preservation of a residual dentition seems to prevent marked changes in jaw-muscle properties (Tallgren et al., 1986). For instance, the cross-sectional areas of the masseter and medial pterygoid muscles in subjects wearing overdentures supported by a small number of teeth were comparable with those of subjects with a natural dentition (Newton et al., 2004). The retention of teeth thus appears to sustain the cross-sectional area of jaw-closing muscles and might therefore enhance masticatory ability.
Subjects with jaw-muscle pain often show severe asymmetrical recruitment of these muscles in comparison with the more symmetrical recruitment seen in normal subjects (Nielsen et al., 1990). Pain that is experimentally induced in the jaw muscles causes a transient, but significant, increase in EMG activities during rest (Svensson et al., 1998b, 2004) and during voluntary contraction, resulting in lower maximum bite forces (Svensson et al., 1998a, 2004). These EMG changes are purely pain related and can be eliminated by local anaesthesia (Yu et al., 1995).
Chronic jaw-muscle pain is a common symptom of CMD. Subjects with myogenous CMD show EMG changes similar to those reported after induction of experimental muscle pain (Buchner et al., 1992). In general, subjects with myogenous CMD try to avoid pain by minimizing both frequency and intensity of voluntary jaw-muscle activity.
It has been suggested that the MyHC composition and cross-sectional area of jaw-muscle fibres are maintained predominantly by powerful contractions exceeding 30 per cent of maximum EMG activity (van Wessel et al., 2005b). Since intense jaw-muscle activity is largely avoided by patients with chronic muscle pain, it is reasonable to assume that their jaw-closing muscles might undergo disuse atrophy, resulting in an increase in the percentage of fibres expressing fast MyHC types and a decrease in the cross-sectional area of slow-type fibres.
Jaw muscles modify their properties in accordance with a changing mechanical environment as they are constantly exposed to various stimuli in the orofacial system. Some of these stimuli are related to therapeutic interventions. On the basis of the approach, it is commonly differentiated between conservative, i.e. non-surgical, and surgical therapeutic interventions. The following section describes the changes in the function and properties of jaw muscles as a response to these interventions, ranging from non-surgical procedures, which might change the mechanical environment more gradually or cause a rather mild response, to more invasive surgical procedures, which have the potential to introduce more significant and sudden changes in the masticatory system.
Oral splints with partial or full coverage of the occlusal surfaces of the teeth in one arch are the most common treatment provided for CMD. It is intended to introduce an optimum functional occlusion that reorganizes the neuromuscular reflex activity, which in turn encourages more normal muscle function. A reduction in jaw-closing muscle activity has been attributed to the effect of these splints (e.g. Lobbezoo et al., 1993; Visser et al., 1995). It has been suggested that elongation of jaw-closing muscles to, or near, the vertical dimension of least EMG activity (the so-called ‘resting zone’) is most effective in producing neuromuscular relaxation (Manns et al., 1983). Although an immediate decrease in EMG activities of the jaw-closing muscles has been reported in numerous studies on oral splints of various vertical dimensions (e.g. Dahlström and Haraldson, 1985; Dahlström et al., 1985; Graham and Rugh, 1988; Visser et al., 1995), there is no evidence for the persistence of this effect in long-term use.
The effects of occlusal splints may be transitory, as it has been reported that, after therapy, the activity of the jaw-closing muscles during clenching on the splint was similar to that measured without the splint (Holmgren et al., 1990; Naeije and Hansson, 1991). Furthermore, comparison of the situations before and after treatment with various types of oral splints yielded no difference in the activity of these muscles during posture and maximal biting in the intercuspal position (Dahlström and Haraldson, 1985; Canay et al., 1998; Hersek et al., 1998). Considering the transience of the effects on muscle activity, it can be assumed that jaw muscles only change their fibre properties in response to treatment with oral splints if they are used to increase the vertical dimension long term.
The initial response to bite opening in the jaw-closing muscles of these species is an increase in their EMG activities (Yaffe et al., 1991). Approximately 1 week later, the muscles start to adapt by changes at the mRNA level (Ohnuki et al., 2000; Arai et al., 2005). These changes are reflected at the protein level 2 weeks into the increase in the vertical dimension: the adaptation comprises an increase in MyHC-I and MyHC-IIA and a corresponding decrease in MyHC-IIB (Sfondrini et al., 1996; Muller et al., 2000). These changes indicate that the muscles respond to the increased stretch by shifting their fibre-type composition towards a higher percentage of slow fibres.
Although bite-opening stretches the jaw-closing muscles, the sarcomere length does not change permanently (Bresin et al., 2000), suggesting that the jaw muscles adapt to the new functional length by increasing the length of their fibres, which involves the production of more sarcomeres in series (Goldspink, 1998).
Individuals wearing removable dentures generally generate less EMG activity of the jaw-closing muscles during posture, masticatory motion, and clenching (Tallgren et al., 1980; Tallgren and Tryde, 1991), as well as less EMG activity of the jaw-opening muscles during maximum opening, than fully dentate subjects (Alajbeg et al., 2006). However, the muscle activity levels, expressed as percentages of the maximum EMG, required to perform mandibular movements are higher in edentulous subjects than in age-matched dentate subjects (Alajbeg et al., 2005, 2006).
After the extraction of teeth and insertion of a full denture, the maximum EMG activities of the jaw-closing muscles initially decrease for up to 6 months (Tallgren et al., 1986). Subsequently, the values increase for up to 2 years, but do not return to pre-extraction levels (Tallgren et al., 1986; Tallgren and Tryde, 1991). The reduced myoelectric output level of the jaw-closing muscles parallels a decreased bite force level. Since maximum bite force is strongly correlated with chewing efficiency, this results in a significant reduction in masticatory function in denture wearers (Fontijn-Tekamp et al., 2000). Although no data are yet available, it is thought that the reduced function leads to atrophic changes in the jaw muscles of denture wearers, which are also reflected in smaller cross-sectional areas and faster MyHC types of their fibres.
In functional jaw orthopaedics, the muscle action of the patient is utilized to produce orthodontic or orthopaedic forces. Using functional appliances to advance the mandible, an attempt is made to treat skeletal malocclusions by altering the functional pattern of the orofacial musculature. The EMG activities of the jaw-closing muscles decrease as an immediate neuromuscular response to mandibular advancement, while those of the jaw-opening muscles increase (Hiyama et al., 2000; Voudouris et al., 2003; Tabe et al., 2005). The EMG activities remain altered only during the first months of treatment; about 6 months into treatment they slowly return to the initial levels in both jaw-closing and jaw-opening muscles (Pancherz and Anehus-Pancherz, 1982; Aggarwal et al., 1999; Hiyama et al., 2000).
Changes in the fibre-type composition of jaw muscles after mandibular advancement have been studied in several animal experiments. Within a few weeks of mandibular advancement, the rat masseter, temporalis, and digastric muscles reduce their number of type IIB fibres in favour of the slower type IIA and IIX fibres (Easton and Carlson, 1990; Sfondrini et al., 1996). Pig masseter, temporalis, and medial pterygoid muscles adapt to mandibular advancement by a transformation of type II into type I fibres (Gedrange et al., 2001a) and an increase in the cross-sectional areas of fibres containing MyHC-I (Gedrange et al., 2001b, 2002). The muscles thus match the altered functional demand by hypertrophy of slow fibres and changes in the fibre-type composition, with transitions mainly inside the fast fibre population, which render the muscles slower.
The surgical correction of skeletal malocclusions involves a variety of manipulations of the facial skeleton. Alteration of the sagittal, vertical, or transverse dimensions of the jaws inevitably involves shortening or lengthening of associated soft tissue structures (Figure 5). The nature, extent, and direction of the surgical jaw displacement largely determine the adaptive reactions in the jaw muscles.
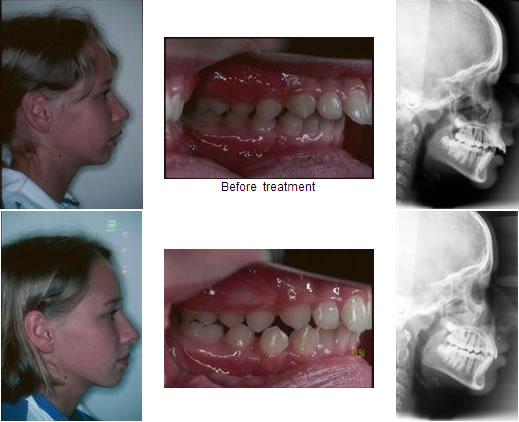
Mandibular repositioning surgery transiently decreases the electric activities and force output of the jaw-closing muscles. After surgery, the patients initially show reduced levels of maximum EMG activities of the temporalis and masseter muscles (Raustia and Oikarinen, 1994; Moss, 1985) and of maximum bite force (Hunt and Cunningham, 1997). These changes are most likely a result of the surgical trauma, the inactivity during intermaxillary fixation, and the anxiety of the patients to clench too hard (Ingervall et al., 1979). In the post-retention phase of surgery, the EMG activities of masseter, temporalis, and lateral pterygoid continuously increase to above pre-operative values with changes continuing to occur at least 1 year after surgery (Moss, 1985). The final values, however, remain lower than those of unoperated control subjects (Harper et al., 1997; Kobayashi et al., 2001; Nakata et al., 2007). The increase is more conspicuous in Class III than in Class II patients (Raustia and Oikarinen, 1994) and is associated with an increase in maximum bite forces (Ellis et al., 1996; Hunt and Cunningham, 1997), masticatory efficiency, and occlusal contact area (Kobayashi et al., 2001). The higher post-operative muscle activity and maximum bite force might thus be a consequence of the improved occlusion.
The cross-sectional areas and volumes of the masseter and medial pterygoid muscles decrease after both mandibular advancement (Dicker et al., 2007) and mandibular setback surgery (Katsumata et al., 2004). In the masseter muscle, the decrease in muscle cross-sectional area and volume parallels a decrease in mRNA encoding MyHC-I and an increase in mRNA encoding MyHC-IIA (Harzer et al., 2007), indicating the beginning of a shift towards a higher percentage of fast fibres. The tendency for recovery of the cross-sectional areas of the jaw-closing muscles (Katsumata et al., 2004) suggests that the changes are attributable to a transient post-surgical atrophy of these muscles. In the long term, however, jaw muscles that are lengthened during mandibular repositioning surgery, such as the digastric during mandibular advancement or the masseter and medial pterygoid during mandibular setback, might undergo significant stretch-induced hypertrophy of their type I fibres (Carlson et al., 1989).
Superior repositioning of the maxilla, performed to correct vertical maxillary excess, also transiently decreases the maximum EMG activities of the jaw-closing muscles. The activities during mastication, however, do not change (Wessberg et al., 1981). The bite forces are reduced initially after surgery, but steadily increase and approach normal values within 2 years (Zarrinkelk et al., 1995; Hunt and Cunningham, 1997). The majority of patients have greater than a 20 per cent increase in occlusal force, which is a larger change than could be accounted for by the altered geometry (Proffit et al., 1989) and might be partially caused by the improved occlusion. The decrease in the mean fibre area and the shift towards a higher percentage of fast fibres in the masseter muscle following superior repositioning of the maxilla (Boyd et al., 1989) are transient and result mainly from disuse atrophy after extended intermaxillary fixation.
Orthognathic surgery thus produces marked alterations in the electric activities, force output levels, volumes, fibre cross-sectional areas, and fibre-type compositions of the jaw-closing muscles with changes continuing to occur at least 1 year after surgery. Changes occurring as a result of post-surgical disuse atrophy are transient and might be followed by a stretch-induced hypertrophy of muscles that are lengthened during surgery (Carlson et al., 1989).
Distraction osteogenesis involves a gradual, controlled displacement of surgically created fractures resulting in a simultaneous expansion of bone and soft tissue. Although this technique has been used for mandibular lengthening in patients with mandibular hypoplasia for more than a decade, information on the associated muscular changes is scarce and, at least in part, appears contradictory. The muscular changes following distraction of the mandible at a rate of 1 mm per day, which is considered optimal for osteogenesis, seem to be muscle specific and include atrophy, hypertrophy, regeneration, and fibrosis (Tüz et al., 2003). Histologic studies have shown a decrease in the mean cross-sectional areas of digastric (Fisher et al., 1997) and masseter (Tüz et al., 2003) muscle fibres after distraction. This atrophy, however, is transient and is associated with pronounced cell proliferation in the stretched muscles, which documents their beginning regeneration (Castano et al., 2001; Sato et al., 2007). The rabbit digastric muscle is completely regenerated after 48 days (Fisher et al., 1997) and the human medial pterygoid muscle after 6 months; the volume sometimes being greater than prior to the distraction (Mackool et al., 2003). The process thus seemingly involves various phases: an initial atrophy of the stretched muscles during and shortly after distraction and a subsequent regeneration in the consolidation period. It has been suggested that any muscle in the same vector of distraction adapts to the elongation with a compensatory regeneration and hypertrophy, whereas muscles lying in a different vector show prolonged evidence of atrophy (Fisher et al., 1997). It also appears that, beyond a certain rate of distraction, the regeneration is insufficient to replace the contractile material that has been damaged by overstretching. Distraction regimens exceeding rates of 2 mm per day, or distances larger than 20 mm, significantly increase the number of damaged sarcomeres (van der Meulen et al., 2005) and cause atrophy, reduced protein synthesis (Fisher et al., 1997), and fibrosis of the muscle (Tüz et al., 2003). The damaged fibres thus undergo degeneration without subsequent regeneration and are replaced by connective tissue.
Fibre-type transformations in leg muscles following distraction osteogenesis resemble those observed in models of muscle overloading (De Deyne et al., 1999). Fibres transiently express MyHC-foetal, indicating regeneration of damaged fibres (Yang et al., 1997). With higher distraction rates, fibres show more evidence of damage and a lower number of fibres expressing foetal myosin (De Deyne et al., 2002). This suggests that the damaged fibres are not regenerated but replaced by connective tissue. Information on changes in the MyHC composition of jaw muscles after mandibular distraction is limited to the rabbit digastric muscle, for which a gradual disappearance of type II fibres with distraction and their reappearance during consolidation have been reported (Sato et al., 2007). It can, however, be assumed that fibre changes in jaw muscles following distraction osteogenesis, similar to those observed in leg muscles, depend on the rate, amount, and vector of distraction.
With regard to orthognathic surgery, the golden rule is that the pterygo-masseteric sling must not be stretched, otherwise relapse is likely to occur. Therefore, the masseter and medial pterygoid muscles are often temporarily detached during mandibular setback surgery in order to reduce post-operative relapse due to muscle pull. In primates, detachment and subsequent reattachment of jaw muscles result in a transient decrease in electric activity (Hohl, 1983), oxidative capacity, and cross-sectional area of type I fibres in these muscles. The capillary density and the fibre-type composition, however, do not change significantly (Maxwell et al., 1981).
When a jaw muscle is detached from its bony insertion without subsequent reattachment, it immediately shortens and often spontaneously reattaches at a length shorter than the original (Yellich et al., 1981). The difference in fibre length is based on a reduction in the number of sarcomeres in series within each fibre (Carlson et al., 1989) to ensure the optimum overlap of the contractile proteins. Interestingly, detachment of muscles does not seem to induce morphologic or histologic changes (Song and Park, 1997), and there is no evidence of atrophy or change in fibre-type composition (Carlson et al., 1989).
In contrast, open reduction with rigid fixation of condylar fractures allows early mobilization. The surgical procedure is followed by a marked decrease in the EMG activities of jaw-closing muscles for several months, which usually returns to pre-operative values after 1 year (Raustia et al., 1997). Bilateral condylar fractures are associated with significantly lower jaw-closing muscle activity during the closing phase (Throckmorton et al., 1999). The reduced bite forces persist for several years after treatment and might be caused by the alteration in craniofacial morphology associated with bilateral condylar fractures: reductions in posterior face height and moment arm lengths for the masseter and pterygoid muscles (Talwar et al., 1998). After unilateral condylar fractures, patients mainly chew on the contralateral side. The increased maximum EMG activities of the masseter and temporalis muscles on this side suggest a partial compensation for the impairments after condylar fracture (Hjorth et al., 1997). One year after treatment, however, the muscle activities do not differ from those of controls. It is, therefore, unlikely that these muscles would alter their cross-sectional areas and fibre-type compositions in the long term.
The surgical resection of the mandibular angle is followed by volume changes in the jaw muscles with the volumes of the masseter and medial pterygoid muscles decreasing up to 4 years post-surgery (Lo et al., 2005). In experiments on rabbits, the atrophy of the masseter was characterized by a loss of muscle mass, a decrease in the mean cross-sectional area of all fibre types, and a decrease in the proportion of type I fibres with a concomitant increase in the proportion of type IIA and IIB fibres (Song and Park, 1997).
Injection of Botulinum toxin A into the masseter muscle leads to a significant reduction in maximum EMG activity for up to 12 months (Lee et al., 2007) as the muscle becomes, at least in part, paralysed (Westgaard and Lømo, 1988). The results are reductions in its functional and energetic capacities (Gedrange et al., 2005c). In order to maintain chewing properties, the function of the paretic muscle is immediately taken over by synergetic jaw muscles, leading to a general increase in their EMG activities (Huang et al., 1993). While parts of the paretic muscle undergo inactivity atrophy (Kwon et al., 2007) and shift their fibre-type composition towards a larger proportion of fast-type fibres, it is conceivable that the parts of the muscles taking over its function adapt to the increased stress by hypertrophy and a shift in their fibre-type composition towards a larger proportion of slow-type fibres.
Jaw muscles are versatile entities capable of changing their size, cross-sectional area, and fibre properties to adapt to altered functional demands. The dynamic nature of muscle fibres allows them to change their phenotype to optimize contractile function and energy uptake. These changes in the phenotype are reflected in differences in the contraction velocity and the maximum force generated by the muscle. Although the adaptive response of muscles follows the same general concept, the changes occurring in individual jaw muscles are not necessarily uniform and vary with the nature of the stimulus. The extent of the adaptations also depends on the quality, intensity, and duration of the stimulus as, for instance, fibre-type transitions exhibit characteristics of a dose-response relationship and only occur under long-term alterations in functional demands. In general, stretch increased neuromuscular activity, and training result in hypertrophy, elicit increases in mitochondrial content, aerobic-oxidative potential, and cross-sectional area, and may change the fibre-type composition of the muscle towards a larger percentage of slow-type fibres. In contrast, changes in the opposite direction occur when neuromuscular activity is reduced; the muscle is immobilized in a shortened position, or paralysed. This might help explain the variability in fibre-type proportions found between and among individuals, muscles, and muscle portions.
Adaptation of jaw closing muscles after surgical mandibular advancement procedures in different vertical craniofacial types: a magnetic resonance imaging study,
The effect of an occlusal stabilization splint and the mode of visual feedback on the activity balance between jaw-elevator muscles during isometric contraction,

Occlusion refers to the relationship between the maxillary and mandibular teeth when they approach each other, as occurs during chewing or rest. Normal occlusion exists when the maxillary incisors just overlap the mandibular incisors (Figure 1A), the mandibular canines are equidistant from the maxillary third incisors and the maxillary canine teeth, and the premolar crown tips of the lower jaw point between the spaces of the upper jaw teeth in a saw-toothed fashion (Figure 1B). Flat-faced breeds, such as boxers, shih tzus, Boston terriers, Lhasa apsos and Persian cats, have abnormal bites that are recognized as normal for their breed in which the mandibular jaw protrudes in front of the maxillary jaw, altering the above tooth-to-tooth relationship (Figures 2A and 2B).
Malocclusion refers to abnormal tooth alignment. Skeletal malocclusion occurs when jaw anomalies result in abnormal jaw alignment that causes the teeth to be out of normal orientation. Dental malposition occurs when jaw alignment is normal but one or more teeth are out of normal orientation.
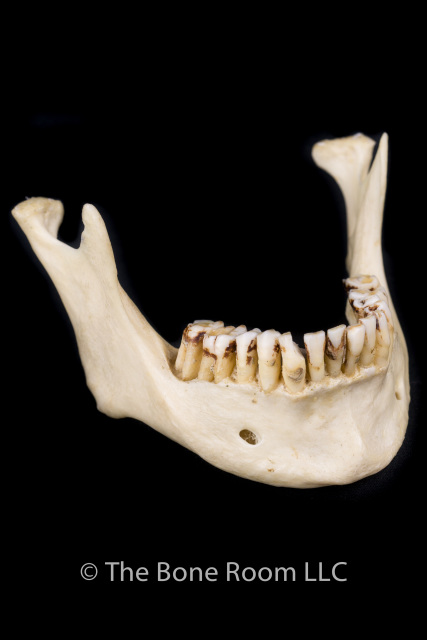
Mandibular distoclusion (also called overbite, overjet, overshot, class 2, and mandibular brachygnathism) occurs when the lower jaw is shorter that the upper and there"s a space between the upper and lower incisors when the mouth is closed. The upper premolars will be displaced rostrally (toward the nose) compared with the lower premolars. Mandibular distoclusion is never normal in any breed (Figures 3A and 3B).
Figure 4. Mandibular mesioclusion in a dog.Maxillary mandibular asymmetry (also called wry bite, especially by breeders) is a skeletal malocclusion in which one side of the jaw grows differently from the other side (Figures 5A and 5B).

An overbite is a genetic, hereditary condition where a dog"s lower jaw is significantly shorter than its upper jaw. This can also be called an overshot jaw, overjet, parrot mouth, class 2 malocclusion or mandibular brachynathism, but the result is the same – the dog"s teeth aren"t aligning properly. In time, the teeth can become improperly locked together as the dog bites, creating even more severe crookedness as the jaw cannot grow appropriately.
Dental examinations for puppies are the first step toward minimizing the discomfort and effects of an overbite. Puppies can begin to show signs of an overbite as early as 8-12 weeks old, and by the time a puppy is 10 months old, its jaw alignment will be permanently set and any overbite treatment will be much more challenging. This is a relatively narrow window to detect and correct overbites, but it is not impossible.
Small overbites often correct themselves as the puppy matures, and brushing the dog"s teeth regularly to prevent buildup can help keep the overbite from becoming more severe. If the dog is showing signs of an overbite, it is best to avoid any tug-of-war games that can put additional strain and stress on the jaw and could exacerbate the deformation.
If an overbite is more severe, dental intervention may be necessary to correct the misalignment. While this is not necessary for cosmetic reasons – a small overbite may look unsightly, but does not affect the dog and invasive corrective procedures would be more stressful than beneficial – in severe cases, a veterinarian may recommend intervention. There are spacers, braces and other orthodontic accessories that can be applied to a dog"s teeth to help correct an overbite. Because dogs" mouths grow more quickly than humans, these accessories may only be needed for a few weeks or months, though in extreme cases they may be necessary for up to two years.
If the dog is young enough, however, tooth extraction is generally preferred to correct an overbite. Puppies have baby teeth, and if those teeth are misaligned, removing them can loosen the jaw and provide space for it to grow properly and realign itself before the adult teeth come in. Proper extraction will not harm those adult teeth, but the puppy"s mouth will be tender after the procedure and because they will have fewer teeth for several weeks or months until their adult teeth have emerged, some dietary changes and softer foods may be necessary.

Canine malocclusion simply refers to when a dog’s teeth don’t fit together properly, whether it’s his baby teeth or adult teeth. Determining whether a dog suffers from malocclusion can be tricky because, unlike with humans, there’s no standard way a dog’s bite should look. “The dimensions and bite configuration of every dog are so different,” says Dr. Santiago Peralta, assistant professor of veterinary dentistry and oral surgery at CUCVM. “The big question is not whether it’s ‘normal,’ but more so: is it functionally comfortable for the animal?”
While breeding can have an impact, there is a range of potential causes for either type of malocclusion. “Malocclusions can have a genetic basis that will be likely transmitted from generation to generation,” Peralta says, “and some of them will be acquired, whether because something happened during gestation or something happened during growth and development, either an infection or trauma or any other event that may alter maxillofacial [face and jaw] growth.” He explains that trauma to the face and jaw can stem from events like being bitten by another animal or getting hit by a car. Fiani adds that jaw fractures that don’t heal properly can also result in malocclusion.
For many of us, orthodontic work – getting fitted with braces, wearing retainers – was just a late-childhood rite of passage. The same went for the pulling of wisdom teeth in early adulthood. Other common conditions, including jaw pain and obstructed sleep apnea – when slack throat muscles interrupt breathing during rest – also just seem like par for the course.
A new study says that parents and caregivers can take steps to promote proper mouth, jawbone and facial musculature development in children to help stave off future health burdens and chronic conditions.(Image credit: Getty Images)
But in a new study, Stanford researchers and colleagues argue that all these issues and more are actually relatively new problems afflicting modern humans and can be traced to a shrinking of our jaws. Moreover, they maintain that this “jaws epidemic” is not primarily genetic in origin, as previously thought, but rather a lifestyle disease. That means the epidemic is largely the result of human practices and akin to obesity, type 2 diabetes, heart disease and some cancers.
The study – published in the journal BioScience – marshals the growing evidence from studies conducted around the world surrounding the jaws epidemic, as well as how to address it proactively. Parents and caregivers can take steps to promote proper mouth, jawbone and facial musculature development in children, the study advises, to help stave off future health burdens and chronic conditions.
“The jaws epidemic is very serious, but the good news is, we can actually do something about it,” said Paul Ehrlich, the Bing Professor of Population Studies, Emeritus, at Stanford and one of the study’s authors.
The new study builds upon a book Ehrlich co-wrote with orthodontist and lead study author Sandra Kahn entitled Jaws: The Story of a Hidden Epidemic, published by Stanford University Press in 2018. Two other Stanford researchers, Robert Sapolsky and Marcus Feldman, have contributed their expertise to the new study. Seng-Mun “Simon” Wong, a general dentist in private practice in Australia, was also a co-author.
Anthropologists have long noted the significant differences between the jaws and teeth in modern skulls compared to pre-agricultural, hunter-gatherer humans from thousands of years ago. The differences are stark even compared to humans who lived as recently as a century-and-a-half ago during pre-industrial times. These bygone humans showed little teeth crowding, impaction of their wisdom teeth (a leading reason for their surgical removal nowadays) or malocclusion – the abnormal positioning of the upper and lower teeth when the mouth is closed.
Paul Ehrlich wants you to shut your mouth – for your health. According to Ehrlich’s new book, mouth breathing, among other modern habits, has led to an epidemic of small jaws and many troubling health consequences.
Assuming that genetics are chiefly responsible for the sudden modern rise of these dental maladies does not make sense, said Ehrlich. “There’s not been enough time for evolution over the span of only several generations to have made our jaws shrink,” said Ehrlich. Nor is there any evidence of selection pressures that would have favored smaller jawed-people producing more offspring – and thus perpetuat




 8613371530291
8613371530291