overshot titration quotation

Your reasoning is correct that $K$ is independent of concentration. But I think you are misreading the question: it wants you to tell how you will miscalculate K with respect to the correct value if you put in the wrong concentrations in the formula, that is, if there was an overshoot in your titration.
Your reasoning is correct that $K$ is independent of concentration. But I think you are misreading the question: it wants you to tell how you will miscalculate K with respect to the correct value if you put in the wrong concentrations in the formula, that is, if there was an overshoot in your titration.
Your reasoning is correct that $K$ is independent of concentration. But I think you are misreading the question: it wants you to tell how you will miscalculate K with respect to the correct value if you put in the wrong concentrations in the formula, that is, if there was an overshoot in your titration.

The endpoint of titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar? Explain.
Titration is a quantitative analytical volumetric technique that permits the determination of the unknown concentration of an analyte with a known concentration of titrant. This is possible because the two react in a known stoichiometric manner allowing calculation of the unknown concentration.

Manual titrations are time consuming and can be inaccurate at times due to human error. Not to mention, important data can get easily lost due to improper tracking methods. The Thermo Scientific Orion Star T900 Series Automated Titrators are designed to make performing titrations easier, more reliable, and more reproducible than manual titrations.
These auto titrators expand the number of ions and compounds that can be measured beyond direct electrode analysis and offer dynamic process controls that adjust the titration to optimize analysis results.
Manual titration can be a time consuming and frustrating process. Watch how easy it is to find the endpoint, reproduce your workflow, and optimize your results. The auto-filling burette helps to minimize the handling of corrosive materials. Use of an auto titrator well help ensure a safer, more efficient lab.
A water treatment plant in the midwestern United States that ran up to 10,000 titrations each year improved their workflow. Learn how streamlining the workflow using an Orion Star Automated Titrator for low-level alkalinity titrations benefited the lab.
In this white paper, you’ll learn about the dispense accuracy and precision of the Orion Star T900 Series Automated Titrators. We’ll demonstrate that our auto titrators exceed well-established industry precision and accuracy specifications, providing users with greater confidence in their titration applications.
Streamline your manual titration workflows and increase efficiency and repeatability with an Orion Star T900 Series Automated Titrator. Review the applications chart below to find out if an auto titrator is for you.
We are currently unable to offer solutions for Karl Fischer, amperometric, stat, and dead stop titrations. For other questions please contact customer support to be connected to your local sales representative.
Get our top 10 tips for performing automated titrations, and methodologies for common uses of an auto titrator. Discover how to perform an acid/base titration for orange juice, water and petroleum in this ebook.
Performing manual titrations can be extremely tedious, requiring the operator to stand in one place, watching minuscule droplets drip into a sample container and diligently waiting for the color change or other endpoint indictor to occur before starting the process all over again, for possibly hours and hours of repeated sample titrations.
An automatic titrator allows you to start the titration and then walk away from the titrator to perform other tasks or tests while the titrator takes care of the titrant addition, endpoint detection and results calculations automatically without any involvement from the operator.
Manual titrations typically use a non-certified, Class B or Class A burette with stopcock to add doses of titrant to the sample. The operator uses the stopcock to start and stop the additions of titrant into the sample, often one drop at a time, until the endpoint is reached.
The precision of these additions, especially near the endpoint, is primarily determined by the operator’s skill level, experience and focus on the task at hand. All too commonly, an operator can allow too much titrant to flow out of the burette into the sample and overshoot the endpoint, requiring them to perform the entire titration all over again. Even highly skilled and experienced operators can be limited in the precision of their manual titration results by the last few drops of titrant, since each drop can vary in volume.
When using an automatic titrator, the titration is performed using a high-accuracy titrant delivery system that controls all titrant additions into the sample and will adjust the dose rate as it detects the endpoint approaching.
Once the endpoint is reached, the titrator uses the precisely measured volume of titrant added to the sample to automatically calculate the concentration results for the sample. The operator’s skill level, experience and focus on the task at hand is no longer a factor in the overall accuracy of the titration results and the possibility of missing the endpoint is greatly reduced.
Typically calculating titrations in the lab is done by calculating the sample concentration after the endpoint has been reached. There are many points in this calculation where human error can get in the way of consistent, reproducible results.
When using an auto titrator, it will automatically calculate the sample concentration from the entered parameters. Plus, on the automatic titrator, electrode, titrant and titration setup parameters can be saved as a method, so the exact same settings are used for each titration. These methods can be transferred between titrators for consistent procedures to be used on multiple titrators or multiple labs. This way you can save time with repeat titrations by running the exact same parameters each time without having to reenter any information.
Instead of manually logging the titration results in a notebook or scrap of paper, an auto titrator will automatically save the titration results in the data log with time and date stamp.
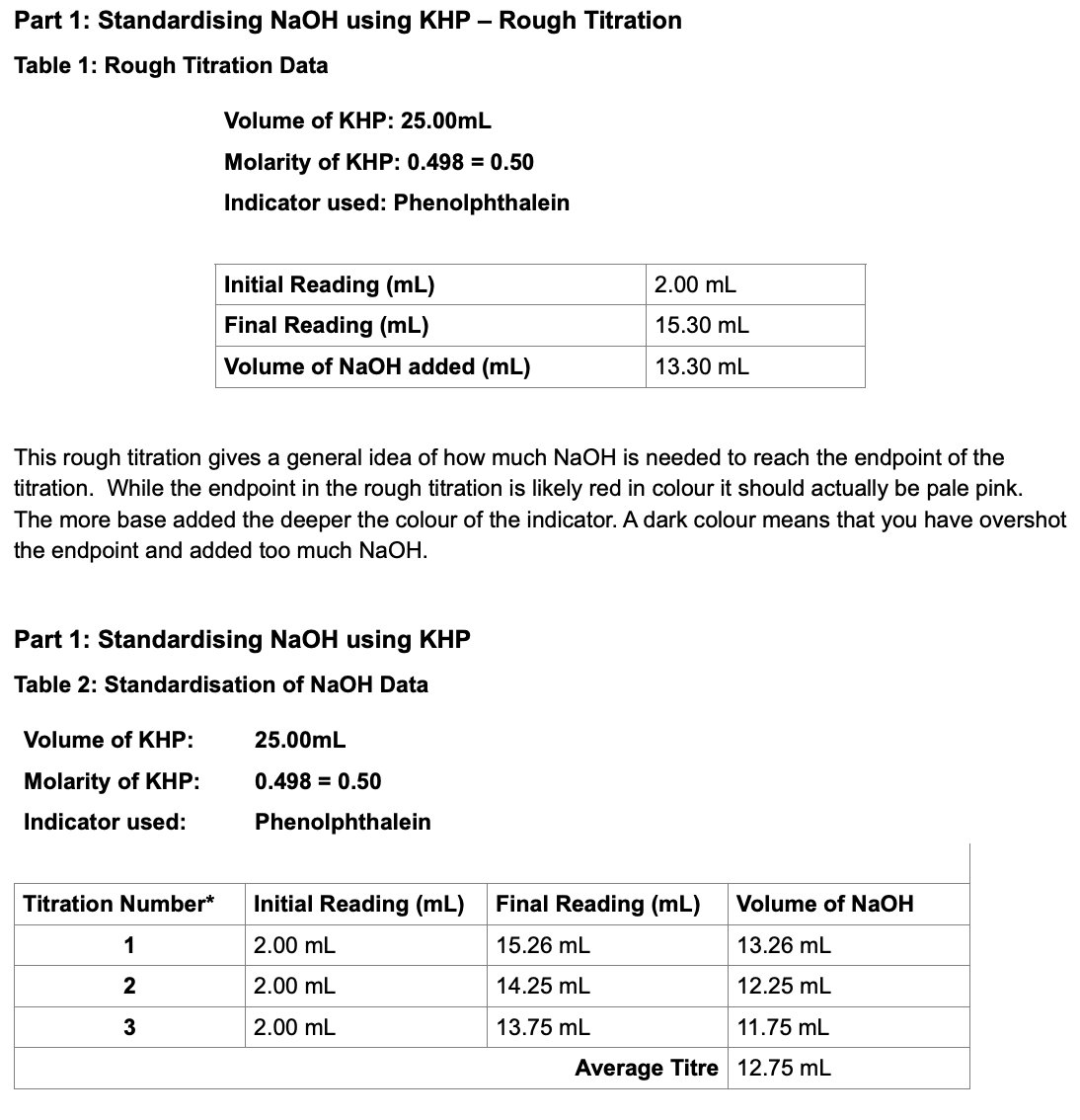
Analysis of items question number 1 right: a balanced equation for the reaction of sodium hydroxide, with corbin acid to form sodium hydrogen a score so we"re first going to start with our orbic acid or vitam and c. Next, we"re going to write an a oh, because that is sodium hydroxide. This will actually form more sodium compound and it will also form water. So we have to make sure this is balanced. We can see na trades places with this 1 h, so our h 2 always balanced, and we have our sodium compound tion to calculate the number of moles of sodium hydroxide that reacted. In order to do this, you need to know the concentration of your n, a o h and the volume used they were not given in this problem. So i"m just going to show a quick example. So let"s say your concentration was 0.015 molarity and the volume used was 25.0 milliliters. The first thing you have to do is convert these milliliters to liters, so that would be 0.025 liter. So then you take your concentration and remember: molarity is just moles over leaders. You multiply by your leaders that cancels out the leaders unit and you will be left with moles, and this comes out to be 3.75 times 10 to the minus 4 moles of n a o h question 3: i calculate the number of moles of escortin acid that Were present in the tablet? Well, if we"re assuming that we"re using this value here, 3.75 times 10 to the minus 4 moles of n a o h, we can use these moles of n a o h to calculate the number of moles of corbin acid. So what we"re going to do is we"re going to look at our balanced chemical equation and we can see that there is a 1 to 1 ratio between n, a o, h and scorbic acid. So this means the number of moles of of an a o h. Should equal the number of moles of scori acid- and you show this by saying for every 1 mole that should be a a oh. You will get 1 mole when you need 1 mole to react with it of a corbi acid, i"m just going to label. This. Will put that up here? So if we have 3.75 times 10 to the minus 4 moles of n, a o h, you will get 3.75 times 10 to the minus 4 moles of your escortin acid question 4 calculate the mass of milligrams of axcorbic acid present in the tablet. So if we have 3.75 times 10 to the minus 4 moles of a a or corbin acid, we can convert these to grams. By saying 1, mole of your corbin acid is equal to the molar mass of this entire compound, and that comes out to be 176.12 grams. This is where we get grams per mole, so we"ll take our previous answer of 3.75 times 10 to the minus 4 and we"ll multiply it by 176.12, and this comes out to be 0.066045 grams. Now it wants it in milligrams. So we"ll say there are 1 gram on the bottom and 1000 milligrams on the top. So we"ll multiply our previous answer by 1000 point, and this comes out to be 66.045 kilograms. Question number 5 determine the percentage of a sportin acid present in the tablet. 1 tablet weighed 0.17 grams. Let"S convert this to milligrams by saying 1000 kilograms for 1 gram, so we"ll take 0.17 multiply that by 1000 point- and this comes out to be 170 kilograms. We want to know the percentage, so we can take 66.045 it now. This is milligrams of your vitamins and 170 kilograms for tablet, so you take 66.045 divided by 170 and then you would get that answer and multiply it by 100 percent and this comes out to be 38.85 percent, so 38, roughly 3839 percent of your tablet is going To be vitamins now, question 6 asks you to do the experiment again with a different indicator, so we"re going to skip down to question 7. How would the percentage of sportin acids in the vitamins tablet be affected if you forgot to fill the tip of the bare with sodium hydroxide before you started with the citation? So what they"re saying is at the very end of the barred down here there was a bit of air and up here was your sodium hydroxide. Now, if you forgot to fill this baret or fill the tip of the buret with sodium hydroxide and just left the air, when you opened the stopper, the sodium hydroxide on the bare would immediately fill the tip of the buret with sodium hydroxide. This would mean your initial reading would actually go down to a different value. So let"s say if your initial reading was 0 milliliters and you filled it up the tip up with an oh and there is exactly 1 milliliter that needed to go into that tip. You would add an extra milliliters into your calculations, but didn"t necessarily have to be there so instead of saying 25.0 milliliters, this would actually become 26.0 milliliters. This would change your calculations now, if i much, but it will change your calculations. So, let"s take a look at what would actually happen or molarity would stay the same. We would still have moles over 1 liter. This time we would multiply it by 0.0260 meters or leaders would cancel, and we would end up with 3.9 times 10 to the minus 4 moles of n a o h. This would equal the same number of moles of our corbin acid. So, let"s find out how many milligrams of scorbic acid we would have at this new polarity, so we have 3.9 times 10 to the minus 4 point as wrackin moles of our scorbic acid. We will multiply that by 176.12 gram for every 1 mole and then multiply that by 1000 kilograms over 1 gram, and in this case we would get 68.6868 milligrams of our escortin acid. So, as you can see, this value is very different, well different than there. Our previous answer, so this is why it is very important to make sure that you calculate your initial starting point for n a o h very accurately, so that you don"t end up with errors carried forward throughout your calculations. Question 8 assumed that the lad partner overshot the end point of the second nitration. How could you salvage the lad without starting over so in this case, you added more n a o h than was needed in this case, the easiest method would be to do an additional trial, so you would just add 1 more trial to your set, and in This instance, let"s say in trial 1, you used 25 milliliters in trial 2. This is the 1 that was overshot. Let"S say you used 27 milliliters for all 3. You used 24.9 milliliters. In this instance, you could see that 27 milliliters is indeed an anomaly. It"S not a strong anomaly, but there is some difference between 2527 and 24.9 point. So then you would just do another trial. You would do trial 4 and then let"s say you came out with 25.1 milliliters. Well in this case you would know that trial 2. There was something wrong with trial 2 and then you can just disregard that trial and then keep going with trials 13 and 4.

Using a color indicator for manual titrations can be fast and simple, but there is a trade-off between time and accuracy. Color indicators are chemical dyes that change color based on the properties of a solution.
These color indicators are often used during manual titrations as an indicator of the consumption of a certain chemical, or the presence of a chemical in excess. The color change correlates to the titration endpoint.
In a potentiometric measuring system, a titration endpoint is determined based on a change in potential in the solution. A meter and sensor accurately determine the millivolt (mV) potential of the sample solution. The sensor, such as a pH, ORP, or ion selective electrode, behaves according to the Nernst equation.
The type of sensor used will determine which ion(s) in the solution are measured. The inner reference potential of the electrode’s cell is compared to the outer membrane potential. During a titration, the activity of the ion being titrated changes as the titration progresses. The titration endpoint can be detected by determining the point where the maximum potential change occurs.
A nickel titration can be determined potentiometrically. However, nickel is a special case in that a sensor to directly detect ion activity is not commercially available. Instead, the nickel concentration can be determined in a titration by monitoring the displacement of copper by nickel with a cupric ion selective electrode (ISE).
We then titrate with EDTA. As the titration progresses, EDTA first binds the nickel ions in solution. Once all the nickel is bound, the EDTA then react with the free copper ions in solution. When this happens, the activity of the copper ion drastically decreases, which is detected by the cupric ISE. This signals the titrator to detect the endpoint.
With potentiometry, we are monitoring the actual activity of the ion we are trying to measure rather than looking at a color change with our eyes. Tracking the titration this way allows the reaction to be monitored in a consistent manner that eliminates subjectivity and increases accuracy and consistency between analysts.
Titrations by hand are tedious and the endpoint can easily be overshot. A manual burette stopcock can only dispense one drop (~50 µL) at a time, and it takes skill to do so. This is not the case with automatic titration. An automatic titrator can dose down to 5 µL per dose with a standard 25 mL burette installed, ensuring that the endpoint is precisely detected every time.
Automation also helps increase accuracy and repeatability without wasting time. By utilizing customizable and flexible dosing options offered by many automatic titrators, the titrator looks at the rate of mV change throughout the titration to determine the dosing speed and size.
By doing this, larger volumes will be dosed more frequently at the beginning of the titration since the potential change is small. As the reaction approaches the endpoint, the mV potential starts to change more dramatically per dose. As a result, the titrator proportionally scales down the dose size and increases the time between doses.
Dosing a larger volume of titrant in the beginning of the titration, and less at the end, keeps the speed of each titration to a minimum while ensuring high resolution around the endpoint. Automating the dosing and endpoint detection allows analysts to perform other lab duties.
Since they used a color indicator, their titration results were open to interpretation between many different technicians. The lab manager noticed that they were obtaining inconsistent results across shifts, and this resulted in inconsistencies in their finished products.
Hanna Instruments worked with the manufacturer to automate their manual titrations using our Automatic Potentiometric Titrator HI932. Once the titrations were automated, results became consistent across shifts and the analysts were able to perform other tasks while a titration was running.
The Automatic Titrator Nickel Package HI932 offers everything you need to accomplish a smooth transition from manual titrations to automated titrations. Our HI932 package includes:

Open Science Framework: Code and Figures for v1 of F1000Research submission: Dose Titration Algorithm Tuning (DTAT) should supersede the Maximum Tolerated Dose (MTD) concept in oncology dose-finding trials, doi
Amendments from Version 1 Changes in v2, stimulated largely by Dr. Natalja Strelkowa"s reviewer comments, follow closely my posted reply. Chiefly, I have sought in v2 to separate the merely incidental aspects of the concrete simulation here presented from the essential content of the DTAT principle. In particular, (a) I now directly contrast DTAT"s dose titration algorithm abstraction against the fallacious abstraction of "the" MTD, introducing the separate acronym "DTA" specifically for added emphasis, and indicating a connection with Karl Popper"s concept of objective knowledge; (b) I stress that recursive filtering methods (such as the Kalman filter) have contributed heuristically to DTAT, but in no way delimit or define DTAT; (c) I discuss at some length (adding 3 new citations) how the DTAT principle applies beyond cytotoxic chemotherapy as simulated here – specifically, to cancer immunotherapies and molecularly targeted agents; (d) I intensify my invective against "the" MTD, directly condemning it as a fallacy of misplaced concreteness, and employing the scare quotes now even in my title. Additionally, Dr. Strelkowa"s very important point about the need for fail-safe upper bounds in DTAs receives stress both in the main text and in the caption to Figure 5. Finally, I strike the suggestions made in v1 that a linear Kalman filter might improve on the Newton-Raphson method used here, since I have realized that the transformations which "linearize" Figure 4 merely simplify, but do not actually linearize, the nadir dynamics. (As I indicate in the text, full-information methods now appear in any case the most promising general line of attack.)
Results.Individual-level myelosuppression dynamics in the simulation model approximately linearize under simple transformations of neutrophil concentration and drug dose. The simulated dose titration exhibits largely satisfactory convergence, with great variance in individualized optimal dosing. Some titration courses exhibit overshooting.
The idea that ‘dose finding studies’ should yield dose titration algorithms (DTAs) is not new. More than a quarter-century ago, Sheiner and colleagues
nadir)-targeted dose titration are then investigated, with an eye to demonstrating the predictability of nadir timing. In particular, an approximate linearization of neutrophil nadir level and timing is demonstrated, achieved through suitable power-law transformation of infusion doses and logarithmic transformation of neutrophil concentration. Within this transformed parameter space, a simple recursive DTA is defined on the basic heuristic of the Newton-Raphson method for root-finding. For simplicity, monitoring of CIN is not modeled endogenously to this algorithm, but is treated as exogenous such that nadir timing and level are known precisely. A ‘DTAT’ study is simulated and visualized for 25 patients, with the
For present purposes, however, it suffices to implement a model-free recursive titration algorithm built on the Newton-Raphson method, with a numerically-estimated derivative based on most recent infusion doses and their corresponding ANC nadirs. In this algorithm, a relaxation factor
at steady state, hysteresis effects arising during initial steps of dose titration do sometimes yield positive numerical estimates for this slope; so the slope estimates are constrained to be ≤ 0. The infusion dose for cycle 1 is 50 mg, and the cycle-2 dose is calculated conservatively using a slope
sequence of titration outcomes emerging in serially enrolled study subjects, it becomes clear that even quite early in the study it will seem desirable to ‘retune’ the titration algorithm. For example, provided that course-1 CIN monitoring is implemented with sufficient intensity to deliver advance warning of an impending severely neutropenic nadir, so that timely colony-stimulating factor may be administered prophylactically
ω downward. Of note, at any given time any such proposed retuning may readily be subjected to a ‘dry run’ using retrospective data from all convergent titration courses theretofore collected. (Hysteresis effects would however be inaccessible to a strictly data-driven dry run absent formal modeling that captures such effects.) Furthermore, the ‘tuning’ idea readily generalizes to the fundamental modification or even wholesale replacement of a DTA. For example, the overshooting seen for subjects
toxicities will generally differ from one patient to another. Dose titration algorithms should most emphatically be tuned to these factors as well. For example, if a patient with more advanced disease and short expected survival nevertheless decided to enroll in a Phase I DTAT study to pursue the possibility of therapeutic benefit, then this patient’s decision would indicate a subjective weighting of benefits vs. harms favoring a
invites scrutiny, supervision and modification by clinical judgment. For example, if during the course of our DTAT study adverse effects other than neutropenia were to emerge as occasional dose-limiting toxicities, then the full concept of ‘tuning’ advanced above would invite dynamic, ‘learn-as-you-go’ modifications of the titration algorithm. Such modifications could begin with decreasing the relaxation factor
supervision and modification by clinical judgment as mentioned above clearly comes to the fore. But even then, the development and application of scoring systems for patient-reported clinical symptoms and quality of life would enable dose titration protocols (DT
optimal control. I have concretely illustrated key elements of DTAT by simulating neutrophil-nadir-targeted titration of a hypothetical cytotoxic chemotherapy drug with pharmacokinetics and myelosuppressive dynamics patterned on previously estimated population models for docetaxel. I have also discussed the applicability of DTAT to other types of anti-cancer therapy. I believe DTAT presents a
Open Science Framework: Code and Figures for v1 of F1000Research submission: Dose Titration Algorithm Tuning (DTAT) should supersede the Maximum Tolerated Dose (MTD) concept in oncology dose-finding trials, doi
Code and Figures for v1 of F1000Research submission: Dose Titration Algorithm Tuning (DTAT) should supersede the Maximum Tolerated Dose (MTD) concept in oncology dose-finding trials.

QuoteOK. This isn"t how a simple titration is done. What"s done is, you add reagent dropwise, either stirring mechanically or swirling manually with each addition. The palest color, that persists with stirring, is the endpoint. You record that volume on paper, and you accept that volume. You use that volume, in a calculation, that tells you how much was there. And that"s what I wanted to see.
A bright purple color is very overshot. You"ll find, once you hit an endpoint like I described, if you add 1 ml more, then 5 ml more, then 10 ml more, no discernible difference in the color. So if you see a bright purple color, you know nothing at all.
Yes, I understand how to do a simple titration, the expected endpoint was to be 8-10mL, instead we got .2-1mL for the endpoint. Which clearly indicates something is wrong, but the reason to is unknown, hence why I am asking this on the forums so that I may gain understanding from others with experience or knowledge of this field to be able to help me.

The Laboratory Charge Analyzer Model LCA-3 is Chemtrac’s newest model lab unit capable of performing automatic titrations to determine optimum coagulant dosage of a raw water sample. The LCA-3 is also capable of determining the amount of pH adjustment chemical, such as lime or caustic, that is needed to maintain optimum pH conditions for coagulation.
The pH is very important to successful coagulant dosage determinations using charge measurement and is therefore an essential feature to the LCA. In this demonstration, the pH of the sample starts at 7.66 but quickly drops with the addition of aluminum sulfate. For this test , the target pH for the treatment process has been defined as 6.3. As the pH is seen to drop below the target pH of 6.3, the LCA begins titrating with caustic to raise pH while being careful to not raise it too quickly or to overshoot the target as this could result in needing to feed excess Alum to obtain charge neutralization. The LCA titration is complete once the charge reading has reached zero and the pH has reached its target value of 6.3. The unit then displays the final result showing the parts-per-million dosage of both chemicals. To establish confidence with the LCA test result, it is recommended to perform jar testing to verify optimum reduction of turbidity and organics. Treatment goals may not call for the level of NTU/TOC reduction that is obtained by full charge neutralization of the sample, in which case correlations can be established between the LCA result and the actual dosages that are required to meet the WTP’s target NTU & TOC reduction. With an accurate measurement of charge neutralization, the LCA-3 provides an affordable and efficient way for water treatment plants to quickly determine an optimum dosage of coagulant.
These analyses have traditionally been performed using a pH meter followed by manual titration with a glass burette; however this approach commonly causes several problems, including overshooting the titration"s endpoint, variation of results between operators and transcription errors.
This automatic titration system, which may be controlled via a PC and user-friendly Tiamo software or by a full-colour touch-control display, operates with the press of a button: the user simply places the sample on a stirrer stand, lowers the pH probe in, then presses the "start" button, which results in completely automated pH reading and titration.

Since I started working at Metrohm more than 15 years ago, I have received many questions about Karl Fischer titration. Some of those questions have been asked repeatedly from several people in different locations around the world. Therefore, I have chosen 20 of the most frequent questions received over the years concerning Karl Fischer equipment and arranged them into three categories: instrument preparation and handling, titration troubleshooting, and the oven technique. Part 1 covered instrument preparation and handling, and Part 2 will now focus on titration troubleshooting and the KF oven technique.
A drift of zero can be a sign that the cell might be over-titrated. In combination with the mV signal (lower than end-point criteria) and the color of the working medium (darker yellow than usual),it is a clear indicator for over-titration. However, volumetric titrations sometimes exhibit a zero drift for a short time without being over-titrated. If you have a real excess of iodine in the titration cell, the result of the next determination will most likely be erroneous. Therefore, over-titration should be avoided. There are various possible reasons for over-titration, like the sample itself (e.g., oxidizing agents which generate iodine from the working medium), the electrode (coating or invisible depositions on the Pt pins/rings), the reagent, and method parameters (e.g., the titration is rate too high), to name just a few.
Different factors can cause over-titration, however, the reagent is not always the reason behind this issue. The indicator electrode can also be the reason for overshooting the endpoint. In this case, regular cleaning of the electrode can prevent over-titration (see also questions 7 to 9 from Part 1 in this series on cleaning).
A low stirring speed also increases the risk of over-titration, so make sure the solution is well mixed. Depending on the type of reagent, the parameters of the titration need to be adjusted. Especially if you use two-component reagents, I recommend decreasing the speed of the titrant addition to avoid over-titration. Over-titration has an influence on the result, especially if the degree of over-titration changes from one determination to the next. So over-titration should always be avoided to guarantee correct results.
I recommend using the drift correction only during coulometric KF titration. You can also use it in volumetric titration, but here the drift level is normally not as stable as for coulometric titrations. This can result in variations in the results. A stabilization time can reduce such an effect. However, compared to the absolute water amounts in volumetry, the influence of drift is usually negligible.
If possible, use a larger sample size to increase the amount of water added to the titration cell with the sample. Furthermore, you should try to reduce the drift value in general. Perhaps the molecular sieve or the septum need to be replaced. You can also use a stabilizing time to make sure the drift is stable before analyzing the sample.
The sample is weighed in a headspace vial and closed with a septum cap. Then the vial is placed in the oven and heated to a predefined temperature, leading the sample to release its water. At the same time, a double hollow needle pierces through the septum. A dry carrier gas, usually nitrogen or dried air, flows into the sample vial. Taking the water of the sample with it, the carrier gas flows into the titration cell where the water content determination takes place.
Very often, the oven is used in combination with a coulometric titrator. The coulometric titration cell used in an oven system is filled with 150 mL of reagent. Theoretically, this amount of reagent allows for the determination of 1500 mg of water. However, this amount is too high to be determined in one titrationand it would lead to very long titration times and negative effects on the results. We recommend that the water content of a single sample (in a vial) should not be higher than 10 mg, ideally around 1000–2000 µg water.For samples with water contents in the higher percentage range, you should consider the combination with a volumetric titrator.




 8613371530291
8613371530291