the endpoint of the titration is overshot price
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

3. In part B if the endpoint of the titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar?

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

During the titration of an HClO4solution with 0.10320M NaOH, a CHEM 3A student,Juan becamedistracted and overshot the endpoint. A fellow student Pedrosuggested that he should record the present volume of NaOH added and titrate the excess with a standard acid solution. If the original sample volume was 25.000mL, the volume of NaOH added was 28.060mL. It took 3.4700 mL of 0.10940 M HCl to back-titrate the NaOH andcalculate the molar concentration of the original HClO4solution.

Hello people. The sample of any work that is 1.0.10 012 Molar has been given to us. The volume is equal to 25. 28.06 Mulan was added to the sample. It took 3.47 liters off of Kolarov. We have to calculate the concentration of original solution. Mhm. An eagle solution. This is the question of backed iteration. The first thing we have to do is calculate the molds of SCL that were added to the poet. The number of molds of steel is equal to 94. We have done more clarity by volume. This will be equal to 0.379612mm. Noel. We have founder moral status of 0.379612 million malls and now they are an ascetic acid, voter mono protic acid and analyzes also mono basic and factor. The liberals will be equal do of based Will be zero 1032 multiplied by 28.06 Minimal which will be equal to 2.9 0.89 five believe board. This is minimal of peace and now many molds of base will be used. What is this? 2.895 793 minutes. 2.51618 Milliman will give us a value of this based on used orvinegar. The amount of base used for aspect asserts will be equal to medieval and acetic acid, which is more clarity compared to volume and it is 2.5 16 18 minimal, which is equal to 25 ML. They will be done modularity from here. The system modularity of original vinegar is equal to 0. 100 64 72 molars. I hope you understood the question. Thank you.

We use cookies and other technologies to improve your experience on our websites. By clicking "Accept", you agree to let us use third-party cookies for analytics purposes. You can learn more about how we use cookies in our Cookies Policy. To manage your cookies, click "Cookies Manager". By clicking consent you confirm you are 16 years or over and agree to our use of cookies.

Manual titrations are time consuming and can be inaccurate at times due to human error. Not to mention, important data can get easily lost due to improper tracking methods. The Thermo Scientific Orion Star T900 Series Automated Titrators are designed to make performing titrations easier, more reliable, and more reproducible than manual titrations.
These auto titrators expand the number of ions and compounds that can be measured beyond direct electrode analysis and offer dynamic process controls that adjust the titration to optimize analysis results.
Manual titration can be a time consuming and frustrating process. Watch how easy it is to find the endpoint, reproduce your workflow, and optimize your results. The auto-filling burette helps to minimize the handling of corrosive materials. Use of an auto titrator well help ensure a safer, more efficient lab.
A water treatment plant in the midwestern United States that ran up to 10,000 titrations each year improved their workflow. Learn how streamlining the workflow using an Orion Star Automated Titrator for low-level alkalinity titrations benefited the lab.
In this white paper, you’ll learn about the dispense accuracy and precision of the Orion Star T900 Series Automated Titrators. We’ll demonstrate that our auto titrators exceed well-established industry precision and accuracy specifications, providing users with greater confidence in their titration applications.
Streamline your manual titration workflows and increase efficiency and repeatability with an Orion Star T900 Series Automated Titrator. Review the applications chart below to find out if an auto titrator is for you.
We are currently unable to offer solutions for Karl Fischer, amperometric, stat, and dead stop titrations. For other questions please contact customer support to be connected to your local sales representative.
Get our top 10 tips for performing automated titrations, and methodologies for common uses of an auto titrator. Discover how to perform an acid/base titration for orange juice, water and petroleum in this ebook.
Performing manual titrations can be extremely tedious, requiring the operator to stand in one place, watching minuscule droplets drip into a sample container and diligently waiting for the color change or other endpoint indictor to occur before starting the process all over again, for possibly hours and hours of repeated sample titrations.
An automatic titrator allows you to start the titration and then walk away from the titrator to perform other tasks or tests while the titrator takes care of the titrant addition, endpoint detection and results calculations automatically without any involvement from the operator.
Manual titrations typically use a non-certified, Class B or Class A burette with stopcock to add doses of titrant to the sample. The operator uses the stopcock to start and stop the additions of titrant into the sample, often one drop at a time, until the endpoint is reached.
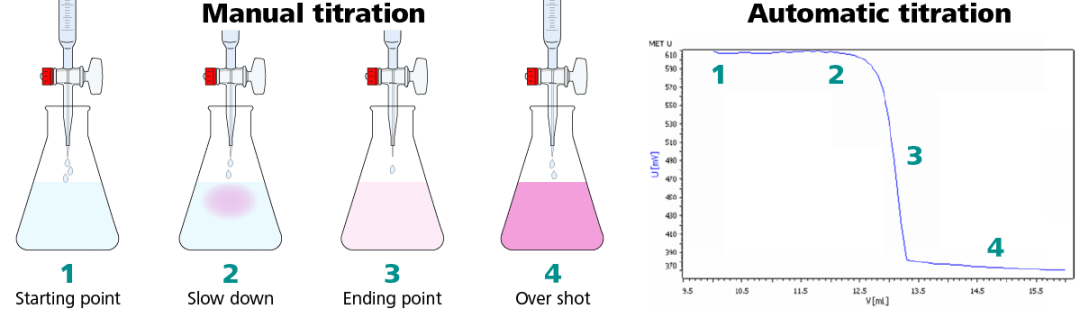
The precision of these additions, especially near the endpoint, is primarily determined by the operator’s skill level, experience and focus on the task at hand. All too commonly, an operator can allow too much titrant to flow out of the burette into the sample and overshoot the endpoint, requiring them to perform the entire titration all over again. Even highly skilled and experienced operators can be limited in the precision of their manual titration results by the last few drops of titrant, since each drop can vary in volume.
When using an automatic titrator, the titration is performed using a high-accuracy titrant delivery system that controls all titrant additions into the sample and will adjust the dose rate as it detects the endpoint approaching.
Once the endpoint is reached, the titrator uses the precisely measured volume of titrant added to the sample to automatically calculate the concentration results for the sample. The operator’s skill level, experience and focus on the task at hand is no longer a factor in the overall accuracy of the titration results and the possibility of missing the endpoint is greatly reduced.
Typically calculating titrations in the lab is done by calculating the sample concentration after the endpoint has been reached. There are many points in this calculation where human error can get in the way of consistent, reproducible results.
When using an auto titrator, it will automatically calculate the sample concentration from the entered parameters. Plus, on the automatic titrator, electrode, titrant and titration setup parameters can be saved as a method, so the exact same settings are used for each titration. These methods can be transferred between titrators for consistent procedures to be used on multiple titrators or multiple labs. This way you can save time with repeat titrations by running the exact same parameters each time without having to reenter any information.
Instead of manually logging the titration results in a notebook or scrap of paper, an auto titrator will automatically save the titration results in the data log with time and date stamp.

An automatic potentiometric titrator pays for itself within the first few weeks of use by removing subjectivity, increasing accuracy, reducing testing times, saving labor, decreasing chemical consumption and costs, and increasing the precision of bath analysis.
Using a color indicator for manual titrations can be fast and simple, but there is a trade-off between time and accuracy. Color indicators are chemical dyes that change color based on the properties of a solution.
These color indicators are often used during manual titrations as an indicator of the consumption of a certain chemical, or the presence of a chemical in excess. The color change correlates to the titration endpoint.
What becomes tricky is where this color change occurs with respect to the eyes of the technician. Questions like “How pink is pink?” or “What shade of orange between yellow and red should I stop at?” are common.
This problem compounds when trying to detect this color change in a colored sample, such as a green nickel bath. These kinds of samples can be challenging even for skilled laboratory analysts to consistently reproduce, and are near impossible for someone who is colorblind.
In a potentiometric measuring system, a titration endpoint is determined based on a change in potential in the solution. A meter and sensor accurately determine the millivolt (mV) potential of the sample solution. The sensor, such as a pH, ORP, or ion selective electrode, behaves according to the Nernst equation.
The type of sensor used will determine which ion(s) in the solution are measured. The inner reference potential of the electrode’s cell is compared to the outer membrane potential. During a titration, the activity of the ion being titrated changes as the titration progresses. The titration endpoint can be detected by determining the point where the maximum potential change occurs.
A nickel titration can be determined potentiometrically. However, nickel is a special case in that a sensor to directly detect ion activity is not commercially available. Instead, the nickel concentration can be determined in a titration by monitoring the displacement of copper by nickel with a cupric ion selective electrode (ISE).
First, the pH of the sample is buffered to approximately pH 10. Next, a small amount of copper EDTA (CuEDTA) is added to the sample. At pH 10, nickel preferentially binds to EDTA, displaces the copper, and results in free copper ions in solution.
We then titrate with EDTA. As the titration progresses, EDTA first binds the nickel ions in solution. Once all the nickel is bound, the EDTA then react with the free copper ions in solution. When this happens, the activity of the copper ion drastically decreases, which is detected by the cupric ISE. This signals the titrator to detect the endpoint.
With potentiometry, we are monitoring the actual activity of the ion we are trying to measure rather than looking at a color change with our eyes. Tracking the titration this way allows the reaction to be monitored in a consistent manner that eliminates subjectivity and increases accuracy and consistency between analysts.
You might ask, “Why is this important?” Well, getting repeatability between shifts and technicians ensures consistent baths and a quality product. The risk of human error is drastically decreased.
Titrations by hand are tedious and the endpoint can easily be overshot. A manual burette stopcock can only dispense one drop (~50 µL) at a time, and it takes skill to do so. This is not the case with automatic titration. An automatic titrator can dose down to 5 µL per dose with a standard 25 mL burette installed, ensuring that the endpoint is precisely detected every time.
Automation also helps increase accuracy and repeatability without wasting time. By utilizing customizable and flexible dosing options offered by many automatic titrators, the titrator looks at the rate of mV change throughout the titration to determine the dosing speed and size.
By doing this, larger volumes will be dosed more frequently at the beginning of the titration since the potential change is small. As the reaction approaches the endpoint, the mV potential starts to change more dramatically per dose. As a result, the titrator proportionally scales down the dose size and increases the time between doses.
Dosing a larger volume of titrant in the beginning of the titration, and less at the end, keeps the speed of each titration to a minimum while ensuring high resolution around the endpoint. Automating the dosing and endpoint detection allows analysts to perform other lab duties.
Hanna Instruments recently worked with a manufacturer at a metal plating facility to automate their bath titration. The quality control analysts were performing manual titrations for the measurement of acidity in the chrome baths, as well as the acidity and nickel in the nickel baths.
Many of the baths had colored sample matrices such as a dark, murky hue, so the customer was forced to use a very small sample size to try and discern their endpoints using color indicators. This is a large facility, so multiple technicians work in the lab.
Since they used a color indicator, their titration results were open to interpretation between many different technicians. The lab manager noticed that they were obtaining inconsistent results across shifts, and this resulted in inconsistencies in their finished products.
This allowed them to run tests more frequently and maintain higher quality baths, while also reducing the amount of rejected finished products. Before, the lab would become backlogged with samples, causing the employees to have to stay late or rush through them. Now, the samples are titrated along with other analyses in a fraction of the time.
The Automatic Titrator Nickel Package HI902 offers everything you need to accomplish a smooth transition from manual titrations to automated titrations. Our HI902 package includes:The HI902 automatic potentiometric titrator with two stirring assemblies and two 40,000-step piston-driven dosing pumps and burettes

A smooth titration graph and receiving results back from your titrator does not always guarantee that they are accurate. Always review your results, your calculations, and quality checks.
It is important when reviewing titration results to ask yourself, “Does this result make sense?”. This is especially the case when switching methodologies, i.e. from manual titrations to automatic titrations. It can be alarming to see an unfamiliar number, but it is important to take a closer look at your specifications to determine the cause of deviation. The four most important parts of a specification to examine are the units, significant digits, the range, and the methodology used to originate the specifications. We will talk about the significance of each.
There are many different ways to represent the results of titration. Units of specifications can vary, but specifications are commonly represented in %, ppm (mg/L or mg/g), or simply mL (milliliters) of titrant used to determine the endpoint. Results can be customized in the method options to match those of the specifications in more cases. Results that vary from the original specification by a common factor, like 10 or a 1000 are most commonly attributed to a difference in units and can be easily adjusted.
The other part of the unit is the analyte, or what specific chemical is being tested. In order to ensure that the results match the specification, it needs to be clear what form of the analyte is being represented.
In the example of a salt titration, some customers prefer that results be represented in sodium chloride, whereas some specifications represent the result in just chloride. The titration is exactly the same for both units, but the calculated results differ because of the difference in molar mass between sodium chloride and chloride. In the case of acidity, titration results are normally represented in units of the predominant acid. A specification that is written in % lactic acid, would differ greatly from a result represented in % citric acid. This is true, not only because of the differences in molar mass, but also the reaction ratio between the titrant and the specific acid. These deviations can easily be corrected in the method so that the results match the specification.
Usually, specifications are written as a range. For example, as discussed above, the sample should contain between 2.0% to 2.5% sodium chloride. Having a wide degree of tolerance in the range of a specification allows some leeway for time savings and sources of error. A very narrow specification range will require good lab technique and adherence to best practices to ensure that the results achieved are accurate.Specifications should be written with these limitations in mind, as well as being based upon the expected concentration of the analyte.The following are the AOAC guidelines for percent recovery and percent relative standard deviation (%RSD) based on the expected analyte concentration.
When possible, it is always a good idea to understand the methodology used to originate the specification. Different methods have varying degrees of accuracy and unique potential interferences. When moving from one methodology to another, i.e. from manual titration to automatic titration, there may be a slight difference in results. In the case of manual titrations, the endpoint is often overshot and the results can vary between operators. This leads to slightly higher numbers than the actual results. Specifications may need to be reevaluated when switching methodologies to improve the accuracy of the specification.
In summary, familiarity with specifications will make interpreting the results of titration much easier. Implementing quality checks, discussed below, will help to ensure that titration results are accurate.
Quality checks are typically used by labs to ensure the accuracy of their reported results. By implementing quality checks into your analysis procedure, you will not only have peace of mind but, you will also have documentation in case the results are called into question. There are several types of quality checks, but we will focus on blanks, laboratory control spikes, duplicates, and matrix spikes.
A blank is a sample of your solvent that is carried through the sample preparation procedure and then titrated to ensure there is no interference or contamination from the analysis procedure. Remember that any titrant that is being dosed is being calculated into the results. Titrant that reacts with your solvent should not be included in sample results and can be factored out in the method options of the titrator.
A Laboratory Control Spike, abbreviated LCS, is a standard of known analyte concentration that is carried through the sample analysis procedure to evaluate the accuracy of the titration method. For example, if we were titrating the concentration of nickel in a plating bath, we would use a nickel standard of verified concentration to validate the accuracy of the testing procedure. The AOAC has published guidelines on percent recovery as it pertains to the concentration of analyte which is listed above. Laboratory Control Spikes can be used to validate new methods or to ensure the continued accuracy of current methods.
A duplicate is a sample that is carried through sample analysis as two independent samples to ensure that the results of the method are repeatable. Often when validating new methods, multiple replicates are analyzed to determine the relative standard deviation (RSD) between samples. The AOAC also has guidelines for %RSD as they pertain to the analyte concentration.
A sample matrix spike, often abbreviated as MS, is a sample that is duplicated and to which a standard of known concentration is added to one of the replicates. Matrix spikes are useful in determining if the other components of your sample are interfering with the methods.
Note: Quality Checks are not limited to the titrator. Pipettes and balances can also be checked using reference material to ensure that they are maintaining their precision and accuracy. Quality checks can be performed as frequently as preferred, but are typically done at least daily or at the beginning of every shift.

This invention generally relates to the quantitative chemical analysis of liquids by means of volumetric titration, more specifically it provides a device that is more compact, rugged, and easier to use than the presently available apparatus, while maintaining accuracy of measurement.
The analysis of fluids for a specific chemical constituent is often accomplished by a procedure known as titration, in which a standard solution is mixed in increments with a sample to which has been added a color-forming indicator so that a marked color change occurs at the point where the amount of standard solution just neutralizes all of the constituent present in the sample. At this endpoint, the amount of the unknown constituent in the sample may be ascertained from the amount of standard solution used.
The prior art basic apparatus used for titration has hardly changed since the beginning, and remains cumbersome and difficult to use. The unknown is delivered to a titration flask with a pipette, then standard is added by means of a burette until the endpoint is reached. Specifically, it suffers from the following disadvantages:
a) The apparatus is of multiple pieces. At a minimum, six pieces are required: a pipette, a burette, a burette stand, a burette clamp, a titration flask, and a funnel.
e) The insides of the pipette and burette must be kept scrupulously clean to avoid drainage errors. This may require the use of dangerous or toxic cleaning agents.
f) The burette must be rinses before use with the standard. This takes time and wastes standard. Also, standard remaining in the burette at the end of a series of titrations must be discarded.
h) Burette measurements are made from the position of the meniscus. The meniscus is curved and is difficult to view. If viewed from an angle, a parallax error may be made
i) A small amount of unknown or standard may be splashed on the side of the titration flask. The titration must be paused to wash down this deposit, or a titration error will occur.
j) Any partial droplet on the tip of the burette is shown by burette reading as having been delivered, but has not been delivered to the titration flask. For best accuracy, it must be washed off into the titration flask.
k) The contents of the flask must be mixed by swirling. Therefore, two hands are required, one to control delivery from the burette, the other to swirl the flask. This may be tiring to the operator.
l) The operator must add precisely the right amount of standard to achieve the endpoint. One must proceed cautiously or too much standard will be added, overshooting the end point. A good deal of time may be consumed doing a titration because of fear of overshooting the end point. This is especially true for an inexperienced operator. If the end point is overshot, the operator must then repeat the titration, or live with a less than optimum result.
n) The apparatus is most accurate when a substantial portion of the contents of the burette is used for a titration. Therefore, to attain the required accuracy, it may be necessary to repeat the titration using a different amount of unknown, either by using a different pipette or by quantitatively diluting the unknown; or it may be necessary to use a standard of greater or lesser strength.
o) It is sometimes advantageous or necessary to perform reverse titrations, where the standard is titrated with the unknown. It is very inconvenient to do a series of reverse titrations, as the burette must be drained and filled with each new unknown.
Improvements have been made by the invention upon the basic apparatus. The glassware may be replaced by plastic. The burette can be arranged so that it is automatically filled. The burette may be replaced by a dispenser with digital readout. Stirring may be done with a magnetic stirrer and stir bar. However, the basic manipulations remain the same, with the result that performing a titration remains a complex and time consuming matter. Simpler methods using drip counting have been described and are used, however, they are of limited accuracy.
Automated analyzers have been developed, however they are expensive and are best used for the analysis of many similar samples. Such analyzers are not suited for field or educational use, or the analysis of a small number of samples. U.S. Pat. No. 5,817,954 issued to Kahng et. al. on Oct. 6, 1998, shows how the apparatus for automatic titration can be simplified, using some of the same ideas as the present patent.
f) No standard is wasted in rinsing the apparatus before use. The apparatus may be made of such a size that lesser amounts of standard and unknown are required, compared to the standard apparatus.
h) The amounts of unknown and standard are read from a ruled scale, with or without vernier, or from a digital display. Reading of the position of a meniscus is eliminated.
l) There is very good indication of the nearness to the endpoint, and the adjustment to the endpoint is rapid and easily done. Therefore, the time required to do titration is much reduced.
q) The apparatus is easily customized or programmed so that the results of titrations of a given sort can be directly read form the scale or digital display, with no computation required. Other objects and advantages will become apparent from the specifications, drawings, and description.
The preferred embodiments of the invention will hereinafter be described in conjunction with the appended drawings, where like designations denote like elements.
A syringe barrel 22 is constructed of material resistant to the chemicals used during the titration. Glass and various plastics are suitable. A needle 30 is attached to the syringe barrel 22 by a fitting, or it may be cemented in place. The needle is generally of stainless steel, but small bore plastic tubing may also be used. The inside bore of the needle should be as small in bore as possible without unduly restricting the uptake and discharge of the standard and unknown. The syringe barrel 22 may also have an opening for attachment of a sensor 44 located at the base of the syringe barrel 22 near the syringe inlet. Placement of the sensor near the inlet is important because when placed there, it can give information about the approach of the endpoint. The sensor is most commonly a pH electrode. Sensor 42 is connected to a meter for sensor 46 which may be an integral part of the titration apparatus. A vernier scale 24 imprinted upon barrel 22 is used in conjunction with a scale 28 imprinted on the plunger 26 to read the volumes used. For less accurate work a vernier is not necessary, and a single mark on the barrel will suffice. Because it is the proportion of unknown to standard that is of interest, the divisions of the scale need not correspond to any standard unit of volume. Rather, they are chosen for maximum readability. Division into centimeters and subdivision into millimeters is a good choice. A displacement sensor 50 connected to volume display 52 may also be used to measure the volumes of unknown and standard. These sensors are in common use, the most basic application being a calipers with digital readout.
The syringe plunger 26 is constructed of materials suitable mechanical and chemical resistant properties. The portion of the plunger that will be in contact with the liquids must be resistant to the chemicals used. Teflon, polyethylene, and polypropylene are suitable materials. The plunger is machined to provide a leak-proof seal to syringe barrel 22, or may have a groove fitted for an o-ring or rings which provide the seal. The tightness of fit of plunger 26 in barrel 22 is sufficient as to prevent inadvertent movement of plunger 26. The plunger may also have a rough surface or rack to be used with a thumb wheel 48 to provide a means of fine movement of the plunger. Small movements of plunger 26 are necessary to get exactly to the endpoint. A magnetic stir bar 32 is located within the syringe.
The magnetic stir bar 32 is spun by drive magnets 34 which are spun by a electric motor 36. Electric motor 36 is powered by a battery 38, and controlled by a switch with speed control 40 or off/on switch 42. Alternately, as known in the art the stir bar is controlled by a plurality of NS switched electromagnets. The stir bar must spin at a controlled rate, suitable as to allow easy addition of standard to the endpoint, as explained below.
A microprocessor with controls and display 58 may be electrically connected to displacement sensor 50. The microprocessor may be used to record the volume information, the strength of the standard, and to calculate the strength of the unknown. A holder 54 may be used to store the apparatus between uses, to charge the battery between uses, and to hold the apparatus in a fixed relationship to the unknown or standard in a beaker 56 during the titration.
The apparatus is first rinsed with water or other suitable liquid and the syringe plunger is positioned at or near the bottom. The small amount of liquid remaining in the syringe will not interfere with the titration. The beginning position is read. The needle tip is then wiped free of any adhering liquid. The syringe is held in a generally horizontal position, and the tip of the needle placed in a sample of the unknown. If the endpoint is to be detected by means of a color change, the addition of a small amount of an indicator to either the standard or unknown is generally necessary. A volume of the unknown is drawn into the syringe. The needle is withdrawn, wiped clean of unknown, and the volume read from the scale and vernier. The stirrer is then turned on. The needle is then placed in a sample of the standard and the standard is drawn up until the endpoint is reached. The rate of stirring is such that mixing is sufficiently slow so the nearness of the endpoint can be easily ascertained, either by a change in color in the region near the inlet or by a change in the sensor readout, the sensor being placed near the inlet. The importance of a proper rate of mixing and how this makes it easy to rapidly adjust to the endpoint cannot be overemphasized. It the mixing rate is too rapid, there will be little notice of the approach of the endpoint. If the mixing rate is too slow, excessive time is spent waiting for mixing to become complete. The small movements necessary to get exactly to the endpoint are more easily made if a thumb wheel or other means is used. At the end of the titration, the amount of standard is read. A calculation using the amount of unknown, the amount of standard, and the strength of the standard is done to give the strength of the unknown. For the most accurate work, a correction for the amount of standard left in the syringe is made. All liquid is expelled from the syringe and the apparatus is ready for the next titration.
If a series of titrations of a given type is planned and a standard of consistent strength is available, a scale may be selected that has a mark showing the amount of unknown to be drawn up, and that will directly read the concentration of the unknown at the end of the titration, making a calculation unnecessary. For example the apparatus can be used to determine the titratable acidity of a wine, or the grape juice or other juice from which a wine is to be made. The syringe is equipped with a mark indicating the amount of unknown to be drawn up. The titration is done with a standard base solution until the endpoint is reached. Marks of the syringe plunger show directly the titratable acidity in any desired units. Thus a series of removable scales can be used with the same plunger to perform different standardized titration.
Thus the reader will see that the titration apparatus of the invention provides a highly compact and easy to use device with many advantages over existing apparatus.
The apparatus is suitable for almost any type of volumetric titration, with the exception of those which evolve a gas, or form a precipitate which would clog the needle. The apparatus as described titrates a liquid with a liquid. A solid can be titrated if it is dissolved and drawn up in its entirety. The apparatus could also find use in the compounding of solutions, especially those that require a titration.
The accuracy attainable is limited principally by the quality of construction and the readability of the volumes. The apparatus will be most accurate when the full volume of the syringe is used, and the amounts of unknown and standard are equal. For example, with a vernier scale and a syringe travel of 70 millimeters, the unknown and the standard could each be read to 0.1 of 35 millimeters, leading to a potential accuracy of about 0.5% for the titration. If the amount of unknown is 10% of the amount of standard, or the reverse, the liquid drawn up in lesser amount could be read to 0.1 of 7 millimeters, leading to a potential accuracy of about 1.4%. Thus, a large range of unknowns can be analyzed with a given standard without great loss of accuracy. If a displacement sensor with digital readout is fitted, the accuracy of reading is increased, as these sensors can detect a change in position of as little as 0.01 millimeter.
While the above description contains many specific features, these should not be construed as limitations on the scope of the invention, but rather as an exemplification of one preferred embodiment thereof. Many other variations are possible. For example, the movement of the plunger could be controlled by means of a motor. The stir bar could be driven by NS switched electromagnets located outside the syringe, either fixed at the bottom of the plunger, or following the movement of the plunger. The needle could be straight, and the syringe operated in a vertical position. The scale could be on the barrel and the vernier on the plunger. The stirrer could be powered by AC current instead of a battery. Accordingly, the scope of the invention should be determined not by the embodiments illustrated, but by the appended claims and their legal equivalents.

This invention relates to the quantitative chemical analysis of liquids by means of volumetric titration, and aims to provide a device that is more compact, rugged, and easier to use than the presently available apparatus, while maintaining accuracy of measurement.
The analysis of fluids for a specific chemical constituent is often accomplished by a procedure known as titration, in which a standard solution is mixed in increments with a sample to which has been added a color-forming indicator so that a marked color change occurs at the point where the amount of standard solution just neutralizes all of the constituent present in the sample. At this endpoint, the amount of the unknown constituent in the sample may be ascertained from the amount of standard solution used.
The basic apparatus used for titrations has hardly changed since the beginning, and remains cumbersome and difficult to use. The unknown is delivered to a titration flask with a pipette, then standard is added by means of a burette until the endpoint is reached. Specifically, it suffers from the following disadvantages:
a) The apparatus is of multiple pieces. At a minimum, six pieces are required: a pipette, a burette, a burette stand, a burette clamp, a titration flask, and a funnel.
e) The insides of the pipette and burette must be kept scrupulously clean to avoid drainage errors. This may require the use of dangerous or toxic cleaning agents.
f) The burette must be rinsed before use with the standard. This takes time and wastes standard. Also, standard remaining in the burette at the end of a series of titrations must be discarded.
h) Burette measurements are made from the position of the meniscus. The meniscus is curved and is difficult to view. If viewed from an angle, a paraflax error may be made.
i) A small amount of unknown or standard may be splashed on the side of the titration flask. The titration must be paused to wash down this deposit, or a titration error will occur.
j) Any partial droplet on the tip of the burette is shown by burette reading as having been delivered, but has not been delivered to the titration flask. For best accuracy, it must be washed off into the titration flask.
k) The contents of the flask must be mixed by swirling. Therefore, two hands are required, one to control delivery from the burette, the other to swirl the flask. This may be tiring to the operator.
l) The operator must add precisely the right of amount of standard to achieve the endpoint. One must proceed cautiously or too much standard will be added, overshooting the endpoint. A good deal of time may be consumed doing a titration because of fear of overshooting the endpoint. This is especially true for an inexperienced operator. If the end point is overshot, the operator must then repeat the titration, or live with a less than optimum result.
n) The apparatus is most accurate when a substantial portion of the contents of the burette is used for a titration. Therefore, to attain the required accuracy, it may be necessary to repeat the titration using a different amount of unknown, either by using a different pipette or by quantitatively diluting the unknown; or it may be necessary to use a standard of greater or lesser strength.
o) It is sometimes advantageous or necessary to perform reverse titrations, where the standard is titrated with the unknown. It is very inconvenient to do a series of reverse titrations, as the buret must be drained and filled with each new unknown.
Improvements have been made upon the basic apparatus. The glassware may be replaced by plastic. The burette can be arranged so that it is automatically filled. The burette may be replace by a dispenser with digital readout. Stirring may be done with a magnetic stirrer and stir bar. However, the basic manipulations remain the same, with the result that performing a titration remains a complex and time consuming matter. Simpler methods using drop counting have been described and are used, however they are of limited accuracy.
Automated analyzers have been developed, however they are expensive and are best used for the analysis of many similar samples. They are not suited for field or educational use, or the analysis of a small number of samples. U.S. Pat. No. 5,817,954 issued to Kahng et. al. on Oct. 6, 1998, shows how the apparatus for automatic titration can be simplified, using some of the same ideas as the present patent.
It is the object of this invention to provide an apparatus and technique for titration that is superior to the existing apparatus. Specifically, some advantages are:
f) No standard is wasted in rinsing the apparatus before use. The apparatus may be made of such a size that lesser amounts of standard and unknown are required, compared to the standard apparatus.
h) The amounts of unknown and standard are read from a ruled scale, with or without vernier, or from a digital display. Reading of the position of a meniscus is eliminated.
l) There is very good indication of the nearness to the endpoint, and the adjustment to the endpoint is rapid and easily done. Therefore, the time required to do a titration is much reduced.
q) The apparatus is easily customized or programed so that the results of titrations of a given sort can be directly read from the scale or digital display, with no computation required.
FIG. 1 shows a section view of one embodiment of the apparatus of the invention; and FIG. 2 shows a perspective view of another embodiment of the invention.
The preferred embodiments of the invention will hereinafter be described in conjunction with the appended drawings, where like designations denote like elements, and in which: 22 syringe barrel 24 vernier scale 26 syringe plunger 28 scale 30 needle 32 magnetic stir bar 34 magnets 36 electric motor 38 battery 40 switch with speed control 42 off/on switch 44 sensor 46 meter for sensor 48 thumb wheel 50 displacement sensor 52 volume display 54 holder 56 beaker 58 microprocessor with controls and display
A syringe barrel 22 is constructed of material resistant to the chemicals used during the titration. Glass and various plastics are suitable. A needle 30 is attached to the syringe barrel 22 by a fitting, or it may be cemented in place. The needle is generally of stainless steel, but small bore plastic tubing may also be used. The inside bore of the needle should be as small in bore as possible without unduly restricting the uptake and discharge of the standard and unknown. The syringe barrel 22 may also have an opening for attachment of a sensor 44, located at the base of the barrel 22 near the syringe inlet. Placement of the sensor near the inlet is important because when placed there, it can give information about the approach of the endpoint. The sensor is most commonly a pH electrode. Sensor 42 is connected to a meter for sensor 46 which may be an integral part of the titration apparatus, A vernier scale 24 imprinted upon barrel 22 is used in conjunction with a scale 28 imprinted on the plunger 26 to read the volumes used. For less accurate work, a vernier is not necessary, and a single mark on the barrel will suffice. Because it is the proportion of unknown to standard that is of interest, the divisions of the scale need not correspond to any standard unit of volume. Rather, they are chosen for maximum readability. Division into centimeters and subdivision into millimeters is a good choice. A displacement sensor 50 connected to volume display 52 may also be used to measure the volumes of unknown and standard. These sensors are in common use, the most basic application being a calipers with digital readout.
The syringe plunger 26 is constructed of materials of suitable mechanical and chemical resistant properties. The portion of the plunger that will be in contact with the liquids must be resistant to the chemicals used. Teflon, polyethylene, and polypropylene are suitable materials. The plunger is machined to provide a leak-proof seal to syringe barrel 22, or may have a groove fitted for an o-ring or rings which provide the seal. The tightness of fit of plunger 26 in barrel 22 is sufficient as to prevent inadvertent movement of plunger 26. The plunger may also have a rough surface or rack to be used with a thumb wheel 48 to provide a means of fine movement of the plunger. Small movements of plunger 26 are necessary to get exactly to the endpoint. A magnetic stir bar 32 is located within the syringe.
The magnetic stir bar 32is spun by drive magnets 34 which are spun by a electric motor 36. Electric motor 36 is powered by a battery 38, and controlled by a switch with speed control 40 or off/on switch 42. Alternately, the stir bar is controlled by a plurality of NS switched electromagnets, a known method. The stir bar must spin at a controlled rate, suitable as to allow easy addition of standard to the endpoint, as explained below.
A microprocessor with controls and display 58 may be electrically connected to displacement sensor 50. The microprocessor may be used to record the volume information, the strength of the standard, and to calculate the strength of the unknown. A holder 54 may be used to store the apparatus between uses, to charge the battery between uses, and to hold the apparatus in a fixed relationship to the unknown or standard in a beaker 56 during the titration.
The apparatus is first rinsed with water or other suitable liquid and the syringe plunger is positioned at or near the bottom. The small amount of liquid remaining in the syringe will not interfere with the titration. The beginning position is read. The needle tip is then wiped free of any adhering liquid. The syringe is held in a generally horizontal position, and the tip of the needle placed in a sample of the unknown. If the endpoint is to be detected by means of a color change, the addition of a small amount of an indicator to either the standard or unknown is generally necessary. A volume of the unknown is drawn into the syringe. The needle is withdrawn, wiped clean of unknown, and the volume read from the scale and vernier. The stirrer is then turned on. The needle is then placed in a sample of the standard and the standard is drawn up until the endpoint is reached. The rate of stirring is such that mixing is sufficiently slow so the the nearness of the endpoint can be easily ascertained, either by a change in color in the region near the inlet. or by a change in the sensor readout, the sensor being placed near the inlet. The importance of a proper rate of mixing and how this makes it easy to rapidly adjust to the endpoint cannot be overemphasized. If the mixing rate is too rapid, there will be little notice of the approach of the endpoint. If the mixing rate is too slow, excessive time is spent waiting for mixing to become complete. The small movements necessary to get exactly to the endpoint are more easily made if a thumb wheel or other means is used. At the end of the titration, the amount of standard is read. A calculation using the amount of unknown, the amount of standard, and the strength of the standard is done to give the strength of the unknown. For the most accurate work, a correction for the amount of standard left in the syringe is made. All liquid is expelled from the syringe and the apparatus is ready for the next titration.
If a series of titrations of a a given type is planned and a standard of consistent strength is available, a scale may be selected that has a mark showing the amount of unknown to be drawn up, and that will directly read the concentration of the unknown at the end of the titration, making a calculation unnecessary. For example the apparatus can be used to determine the titratable acidity of a wine, or the grape juice or other juice from which a wine is to be made. The syringe is equipped with a mark indicating the amount of unknown to be drawn up. The titration is done with a standard base solution until the endpoint is reached. Marks on the syringe plunger show directly the titratable acidity in any desired units. Thus a series of removable scales can be used with the same plunger to perform different standardized titrations.
Thus the reader will see that the titration apparatus of the invention provides a highly compact and easy to use device with many advantages over existing apparatus.
The apparatus is suitable for almost any type of volumetric titration, with the exception of those which evolve a gas, or form a precipitate which would clog the needle. The apparatus as described titrates a liquid with a liquid. A solid can be titrated if it is dissolved and drawn up in its entirety. The apparatus could also find use in the compounding of solutions, especially those that require a titration.
The accuracy attainable is limited principally by the quality of construction and the readability of the volumes. The apparatus will be most accurate when the full volume of the syringe is used, and the amounts of unknown and standard are equal. For example, with a vernier scale and a syringe travel of 70 millimeters, the unknown and the standard could each be read to 0.1 of 35 millimeters, leading to a potential accuracy of about 0.5% for the titration. If the amount of unknown is 10% of the amount of the standard, or the reverse, the liquid drawn up in lesser amount could be read to 0.1 of 7 millimeters, leading to a potential accuracy of about 1.4%. Thus, a large range of unknowns can be analyzed with a given standard without great loss of accuracy. If a displacement sensor with digital readout is fitted, the accuracy of reading is increased, as these sensors can detect a change in position of as little as 0.01 millimeter.
While the above description contains many specificities, these should not be construed as limitations on the scope of the invention, but rather as an exemplification of one preferred embodiment thereof. Many other variations are possible. For example, the movement of the plunger could be controlled by means of a motor. The stir bar could be driven by NS switched electromagnets located outside the syringe, either fixed at the bottom of the plunger, or following the movement of the plunger. The needle could be straight, and the syringe operated in a vertical position. The scale could be on the barrel and the vernier on the plunger. The stirrer could be powered by AC current instead of a battery. Accordingly, the scope of the invention should be determined not by the embodiments illustrated, but by the appended claims and their legal equivalents.




 8613371530291
8613371530291