the endpoint of the titration is overshot quotation
The endpoint of titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar? Explain.
Titration is a quantitative analytical volumetric technique that permits the determination of the unknown concentration of an analyte with a known concentration of titrant. This is possible because the two react in a known stoichiometric manner allowing calculation of the unknown concentration.
This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

Hello everyone. So for this question we have been given a standard iteration of this key HP with sodium hydroxide. That is a new age and the indicators culture change persists longer. And we are approaching the end point of this citation. So at this point of time, what should we do? And these are the four given statements that we do know which of these is the correct statement. So first of all, what is this? K HP. So K HP is protection hydrogen talic and this is a weak acid that is commonly used. Standard for titillation with sodium hydroxide. Now one mole of this protection hydrogen palette that is K HP is neutralized By one mole of sodium hydroxide. So this is a basic arrangement in which there is a purity which contains this sodium hydroxide and this beaker contains this K HP solution. Now what happens is that into this K HP solution? We add some few drops of any indicator that is suitable. So the indicator is added because when all of the asset that is this K HP will be neutralized. The solution will become slightly basic and it will be indicated by the culture change of this indicator. And since then point is approaching we should add an image in small increments because if more of the animals is added then the solution will become highly basic in a very short period of time that is the over shooting of the endpoint will occur and we will have incorrect values for the dedication of this unknown sodium hydroxide solution. Therefore we can write that option B will be the correct answer. So that is it for the question. I hope everything is clear to you. Thank you for watching.

Analysis of items question number 1 right: a balanced equation for the reaction of sodium hydroxide, with corbin acid to form sodium hydrogen a score so we"re first going to start with our orbic acid or vitam and c. Next, we"re going to write an a oh, because that is sodium hydroxide. This will actually form more sodium compound and it will also form water. So we have to make sure this is balanced. We can see na trades places with this 1 h, so our h 2 always balanced, and we have our sodium compound tion to calculate the number of moles of sodium hydroxide that reacted. In order to do this, you need to know the concentration of your n, a o h and the volume used they were not given in this problem. So i"m just going to show a quick example. So let"s say your concentration was 0.015 molarity and the volume used was 25.0 milliliters. The first thing you have to do is convert these milliliters to liters, so that would be 0.025 liter. So then you take your concentration and remember: molarity is just moles over leaders. You multiply by your leaders that cancels out the leaders unit and you will be left with moles, and this comes out to be 3.75 times 10 to the minus 4 moles of n a o h question 3: i calculate the number of moles of escortin acid that Were present in the tablet? Well, if we"re assuming that we"re using this value here, 3.75 times 10 to the minus 4 moles of n a o h, we can use these moles of n a o h to calculate the number of moles of corbin acid. So what we"re going to do is we"re going to look at our balanced chemical equation and we can see that there is a 1 to 1 ratio between n, a o, h and scorbic acid. So this means the number of moles of of an a o h. Should equal the number of moles of scori acid- and you show this by saying for every 1 mole that should be a a oh. You will get 1 mole when you need 1 mole to react with it of a corbi acid, i"m just going to label. This. Will put that up here? So if we have 3.75 times 10 to the minus 4 moles of n, a o h, you will get 3.75 times 10 to the minus 4 moles of your escortin acid question 4 calculate the mass of milligrams of axcorbic acid present in the tablet. So if we have 3.75 times 10 to the minus 4 moles of a a or corbin acid, we can convert these to grams. By saying 1, mole of your corbin acid is equal to the molar mass of this entire compound, and that comes out to be 176.12 grams. This is where we get grams per mole, so we"ll take our previous answer of 3.75 times 10 to the minus 4 and we"ll multiply it by 176.12, and this comes out to be 0.066045 grams. Now it wants it in milligrams. So we"ll say there are 1 gram on the bottom and 1000 milligrams on the top. So we"ll multiply our previous answer by 1000 point, and this comes out to be 66.045 kilograms. Question number 5 determine the percentage of a sportin acid present in the tablet. 1 tablet weighed 0.17 grams. Let"S convert this to milligrams by saying 1000 kilograms for 1 gram, so we"ll take 0.17 multiply that by 1000 point- and this comes out to be 170 kilograms. We want to know the percentage, so we can take 66.045 it now. This is milligrams of your vitamins and 170 kilograms for tablet, so you take 66.045 divided by 170 and then you would get that answer and multiply it by 100 percent and this comes out to be 38.85 percent, so 38, roughly 3839 percent of your tablet is going To be vitamins now, question 6 asks you to do the experiment again with a different indicator, so we"re going to skip down to question 7. How would the percentage of sportin acids in the vitamins tablet be affected if you forgot to fill the tip of the bare with sodium hydroxide before you started with the citation? So what they"re saying is at the very end of the barred down here there was a bit of air and up here was your sodium hydroxide. Now, if you forgot to fill this baret or fill the tip of the buret with sodium hydroxide and just left the air, when you opened the stopper, the sodium hydroxide on the bare would immediately fill the tip of the buret with sodium hydroxide. This would mean your initial reading would actually go down to a different value. So let"s say if your initial reading was 0 milliliters and you filled it up the tip up with an oh and there is exactly 1 milliliter that needed to go into that tip. You would add an extra milliliters into your calculations, but didn"t necessarily have to be there so instead of saying 25.0 milliliters, this would actually become 26.0 milliliters. This would change your calculations now, if i much, but it will change your calculations. So, let"s take a look at what would actually happen or molarity would stay the same. We would still have moles over 1 liter. This time we would multiply it by 0.0260 meters or leaders would cancel, and we would end up with 3.9 times 10 to the minus 4 moles of n a o h. This would equal the same number of moles of our corbin acid. So, let"s find out how many milligrams of scorbic acid we would have at this new polarity, so we have 3.9 times 10 to the minus 4 point as wrackin moles of our scorbic acid. We will multiply that by 176.12 gram for every 1 mole and then multiply that by 1000 kilograms over 1 gram, and in this case we would get 68.6868 milligrams of our escortin acid. So, as you can see, this value is very different, well different than there. Our previous answer, so this is why it is very important to make sure that you calculate your initial starting point for n a o h very accurately, so that you don"t end up with errors carried forward throughout your calculations. Question 8 assumed that the lad partner overshot the end point of the second nitration. How could you salvage the lad without starting over so in this case, you added more n a o h than was needed in this case, the easiest method would be to do an additional trial, so you would just add 1 more trial to your set, and in This instance, let"s say in trial 1, you used 25 milliliters in trial 2. This is the 1 that was overshot. Let"S say you used 27 milliliters for all 3. You used 24.9 milliliters. In this instance, you could see that 27 milliliters is indeed an anomaly. It"S not a strong anomaly, but there is some difference between 2527 and 24.9 point. So then you would just do another trial. You would do trial 4 and then let"s say you came out with 25.1 milliliters. Well in this case you would know that trial 2. There was something wrong with trial 2 and then you can just disregard that trial and then keep going with trials 13 and 4.

This website is using a security service to protect itself from online attacks. The action you just performed triggered the security solution. There are several actions that could trigger this block including submitting a certain word or phrase, a SQL command or malformed data.

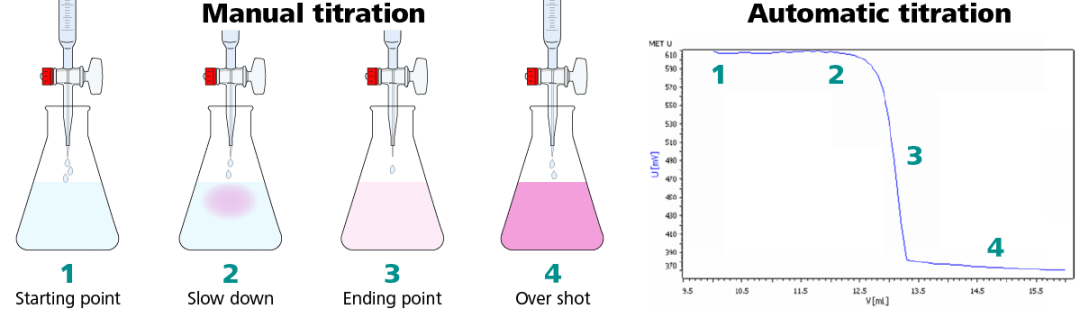
Using a color indicator for manual titrations can be fast and simple, but there is a trade-off between time and accuracy. Color indicators are chemical dyes that change color based on the properties of a solution.
These color indicators are often used during manual titrations as an indicator of the consumption of a certain chemical, or the presence of a chemical in excess. The color change correlates to the titration endpoint.
What becomes tricky is where this color change occurs with respect to the eyes of the technician. Questions like “How pink is pink?” or “What shade of orange between yellow and red should I stop at?” are common.
This problem compounds when trying to detect this color change in a colored sample, such as a green nickel bath. These kinds of samples can be challenging even for skilled laboratory analysts to consistently reproduce, and are near impossible for someone who is colorblind.
In a potentiometric measuring system, a titration endpoint is determined based on a change in potential in the solution. A meter and sensor accurately determine the millivolt (mV) potential of the sample solution. The sensor, such as a pH, ORP, or ion selective electrode, behaves according to the Nernst equation.
The type of sensor used will determine which ion(s) in the solution are measured. The inner reference potential of the electrode’s cell is compared to the outer membrane potential. During a titration, the activity of the ion being titrated changes as the titration progresses. The titration endpoint can be detected by determining the point where the maximum potential change occurs.
A nickel titration can be determined potentiometrically. However, nickel is a special case in that a sensor to directly detect ion activity is not commercially available. Instead, the nickel concentration can be determined in a titration by monitoring the displacement of copper by nickel with a cupric ion selective electrode (ISE).
First, the pH of the sample is buffered to approximately pH 10. Next, a small amount of copper EDTA (CuEDTA) is added to the sample. At pH 10, nickel preferentially binds to EDTA, displaces the copper, and results in free copper ions in solution.
We then titrate with EDTA. As the titration progresses, EDTA first binds the nickel ions in solution. Once all the nickel is bound, the EDTA then react with the free copper ions in solution. When this happens, the activity of the copper ion drastically decreases, which is detected by the cupric ISE. This signals the titrator to detect the endpoint.
With potentiometry, we are monitoring the actual activity of the ion we are trying to measure rather than looking at a color change with our eyes. Tracking the titration this way allows the reaction to be monitored in a consistent manner that eliminates subjectivity and increases accuracy and consistency between analysts.
You might ask, “Why is this important?” Well, getting repeatability between shifts and technicians ensures consistent baths and a quality product. The risk of human error is drastically decreased.
Titrations by hand are tedious and the endpoint can easily be overshot. A manual burette stopcock can only dispense one drop (~50 µL) at a time, and it takes skill to do so. This is not the case with automatic titration. An automatic titrator can dose down to 5 µL per dose with a standard 25 mL burette installed, ensuring that the endpoint is precisely detected every time.
Automation also helps increase accuracy and repeatability without wasting time. By utilizing customizable and flexible dosing options offered by many automatic titrators, the titrator looks at the rate of mV change throughout the titration to determine the dosing speed and size.
By doing this, larger volumes will be dosed more frequently at the beginning of the titration since the potential change is small. As the reaction approaches the endpoint, the mV potential starts to change more dramatically per dose. As a result, the titrator proportionally scales down the dose size and increases the time between doses.
Dosing a larger volume of titrant in the beginning of the titration, and less at the end, keeps the speed of each titration to a minimum while ensuring high resolution around the endpoint. Automating the dosing and endpoint detection allows analysts to perform other lab duties.
Many of the baths had colored sample matrices such as a dark, murky hue, so the customer was forced to use a very small sample size to try and discern their endpoints using color indicators. This is a large facility, so multiple technicians work in the lab.
Since they used a color indicator, their titration results were open to interpretation between many different technicians. The lab manager noticed that they were obtaining inconsistent results across shifts, and this resulted in inconsistencies in their finished products.
Hanna Instruments worked with the manufacturer to automate their manual titrations using our Automatic Potentiometric Titrator HI932. Once the titrations were automated, results became consistent across shifts and the analysts were able to perform other tasks while a titration was running.
This allowed them to run tests more frequently and maintain higher quality baths, while also reducing the amount of rejected finished products. Before, the lab would become backlogged with samples, causing the employees to have to stay late or rush through them. Now, the samples are titrated along with other analyses in a fraction of the time.
The Automatic Titrator Nickel Package HI932 offers everything you need to accomplish a smooth transition from manual titrations to automated titrations. Our HI932 package includes:

Manual titrations are time consuming and can be inaccurate at times due to human error. Not to mention, important data can get easily lost due to improper tracking methods. The Thermo Scientific Orion Star T900 Series Automated Titrators are designed to make performing titrations easier, more reliable, and more reproducible than manual titrations.
These auto titrators expand the number of ions and compounds that can be measured beyond direct electrode analysis and offer dynamic process controls that adjust the titration to optimize analysis results.
Manual titration can be a time consuming and frustrating process. Watch how easy it is to find the endpoint, reproduce your workflow, and optimize your results. The auto-filling burette helps to minimize the handling of corrosive materials. Use of an auto titrator well help ensure a safer, more efficient lab.
A water treatment plant in the midwestern United States that ran up to 10,000 titrations each year improved their workflow. Learn how streamlining the workflow using an Orion Star Automated Titrator for low-level alkalinity titrations benefited the lab.
In this white paper, you’ll learn about the dispense accuracy and precision of the Orion Star T900 Series Automated Titrators. We’ll demonstrate that our auto titrators exceed well-established industry precision and accuracy specifications, providing users with greater confidence in their titration applications.
Streamline your manual titration workflows and increase efficiency and repeatability with an Orion Star T900 Series Automated Titrator. Review the applications chart below to find out if an auto titrator is for you.
We are currently unable to offer solutions for Karl Fischer, amperometric, stat, and dead stop titrations. For other questions please contact customer support to be connected to your local sales representative.
Get our top 10 tips for performing automated titrations, and methodologies for common uses of an auto titrator. Discover how to perform an acid/base titration for orange juice, water and petroleum in this ebook.
Performing manual titrations can be extremely tedious, requiring the operator to stand in one place, watching minuscule droplets drip into a sample container and diligently waiting for the color change or other endpoint indictor to occur before starting the process all over again, for possibly hours and hours of repeated sample titrations.
An automatic titrator allows you to start the titration and then walk away from the titrator to perform other tasks or tests while the titrator takes care of the titrant addition, endpoint detection and results calculations automatically without any involvement from the operator.
Manual titrations typically use a non-certified, Class B or Class A burette with stopcock to add doses of titrant to the sample. The operator uses the stopcock to start and stop the additions of titrant into the sample, often one drop at a time, until the endpoint is reached.
The precision of these additions, especially near the endpoint, is primarily determined by the operator’s skill level, experience and focus on the task at hand. All too commonly, an operator can allow too much titrant to flow out of the burette into the sample and overshoot the endpoint, requiring them to perform the entire titration all over again. Even highly skilled and experienced operators can be limited in the precision of their manual titration results by the last few drops of titrant, since each drop can vary in volume.
When using an automatic titrator, the titration is performed using a high-accuracy titrant delivery system that controls all titrant additions into the sample and will adjust the dose rate as it detects the endpoint approaching.
Once the endpoint is reached, the titrator uses the precisely measured volume of titrant added to the sample to automatically calculate the concentration results for the sample. The operator’s skill level, experience and focus on the task at hand is no longer a factor in the overall accuracy of the titration results and the possibility of missing the endpoint is greatly reduced.
Typically calculating titrations in the lab is done by calculating the sample concentration after the endpoint has been reached. There are many points in this calculation where human error can get in the way of consistent, reproducible results.
When using an auto titrator, it will automatically calculate the sample concentration from the entered parameters. Plus, on the automatic titrator, electrode, titrant and titration setup parameters can be saved as a method, so the exact same settings are used for each titration. These methods can be transferred between titrators for consistent procedures to be used on multiple titrators or multiple labs. This way you can save time with repeat titrations by running the exact same parameters each time without having to reenter any information.
Instead of manually logging the titration results in a notebook or scrap of paper, an auto titrator will automatically save the titration results in the data log with time and date stamp.

1.A known quantity of the unknown solution (HCl) is pipetted into a flask and several drops of an indicator are added. If phenolphthalein is being used as an indicator, the solution should remain colorless at this point. The flask is placed on white paper to make the endpoint easier to see.
2.Make sure the buret stopcock is closed and then rinse the inside with several milliliters of titrant (NaOH). The buret should be held nearly horizontally and rotated so that all of the inside surfaces are contacted by the titrant. Some titrant should also be run through the stopcock to clean it as well. Cleaning is normally performed over a sink.
3.Make sure the stopcock is closed. Place the buret in a buret clamp and fill it carefully with titrant. Use a beaker with a spout or funnel to reduce the possibility of spilling titrant.
5.Read the volume of the buret. This is your initial volume (14.62 ml in this case). Reading is made easier by holding a piece of dark paper behind the buret.
6.Place the flask containing the unknown under the buret. Slowly open the stopcock and add some titrant (usually a milliliter or so). You may notice a temporary color change in the solution near where the titrant was added. Stir the solution thoroughly. Any color change should disappear.
7.Continue adding titrant in small quantities. As the titration progresses, the color change described in step 6 will take longer to disappear. This signals that the endpoint is getting closer and that the titrant should be added in smaller and smaller quantities. Titrant should be added dropwise very close to the endpoint.
8.The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds). It is possible to overshoot the endpoint by adding too much titrant. A correct endpoint is shown on the left, an overshot endpoint on the right.
9.Record the volume in the buret. This is your final volume (26.48 ml in this case). Subtract the initial volume (step 5) from the final volume to determine the volume of titrant added (26.48 - 14.62 = 11.76 ml).

The Purpose of this lab was to determine how much Hydrogen Peroxide is remaining after a reaction containing no catalase (baseline) and catalase (timed trials).
I want to know why, when using Potassium Permanganate to find the amount of Hydrogen Peroxide remaining, that after 1-3 drops, the solution turns pink.
I am a high school student who does not have access to half of these chemicals or supplies, so most of the responses I type are going to be simulated in my mind sadly.
QuoteWhat would you expect, if there was no peroxide, as in plain water, or with catalase added? What would you expect for exactly half degraded peroxide?QuoteThis isn"t how a simple titration is done.
Again, what exactly are you wanting from me by stating "expect." There are many things I can expect from observing degraded hydrogen peroxide. Noticeably that it looks the same as new hydrogen peroxide and reacts less strongly. It decomposes very slowly into Water and Oxygen gas and in the presence of a catalyst, the reaction becomes more noticeable giving off lots of oxygen gas.
QuoteOK. This isn"t how a simple titration is done. What"s done is, you add reagent dropwise, either stirring mechanically or swirling manually with each addition. The palest color, that persists with stirring, is the endpoint. You record that volume on paper, and you accept that volume. You use that volume, in a calculation, that tells you how much was there. And that"s what I wanted to see.
A bright purple color is very overshot. You"ll find, once you hit an endpoint like I described, if you add 1 ml more, then 5 ml more, then 10 ml more, no discernible difference in the color. So if you see a bright purple color, you know nothing at all.
Yes, I understand how to do a simple titration, the expected endpoint was to be 8-10mL, instead we got .2-1mL for the endpoint. Which clearly indicates something is wrong, but the reason to is unknown, hence why I am asking this on the forums so that I may gain understanding from others with experience or knowledge of this field to be able to help me.
QuoteThere is nothing quantitative in this statement. And that makes me sad. I had such high hopes at the beginning of the paragraph, and then you describe the reaction from the textbook, instead of what you saw. Highly disappointing.
Quantitative... I do not recall being taught how to gather quantitative or qualitative data in any of my high school classes, so this may be a significant disadvantage to the responses you will get from me. Upon doing a quick reading, I assume two things that you wanted from me: Numbers and Calculations. However, what was needed to be calculated?
If you want anything else, please explain to me what you want and if there are specific conditions. The terms used, "what was expected" and "what you would expect..." are quite ambiguous and I assumed from your original statements, that if I had a beaker of plain water (with or without sulfuric acid) and no peroxide, what would happen if I added KMnO4 to it. Either way, titrating or adding KMnO4 to that solution wouldn"t necessarily change color, I believe; if I am wrong please correct me. Please also keep in mind that I am new to these forums and do not know how responses are wanted here, so clarification would be great.
Sorry for the excessive quoting at the beginning, but it was unclear what was needed from me, however I feel as it is mostly my fault as I did not ask beforehand for clarification.
Edit: If its worth mentioning, my teachers, a couple of seniors and I did a bunch of experiments after class that same day changing different variables in the experiment. Some of which included, changing the molarity of the chemicals, sequence of the chemicals added, and time for the reaction to take place or to be left undisturbed before titrating. All of which ended in the same result, a 5mL sample extracted from the 21mL sample turning pink within 1-3 drops. I also have a link to the lab we did if you need it.

It’s important to be realistic, sometimes we overshoot. We’ll be like, ‘Let’s go on this six-mile hike’ and expect Roam to be an angel for a six-mile hike… Just know that there are probably going to be meltdowns in the process at some point.
So can further evidence that the FOMC’s outlook for inflation is shifting. In particular, analysts will look for signs that the FOMC is less confident the spike in inflation will be transitory, or that the FOMC’s tolerance for an inflation overshoot is waning.
Since the inner clock relies on sunlight to stay in sync, winter sunrise is later and winter nights are longer, melatonin can overshoot into the day, causing grogginess or "brain fog," for several hours.

“Threats against Muslim Americans are up,” the senator said. “Threats against Sikh Americans are up. Threats against Black Americans are up. Threats against Asian Americans are up. But the number one religious group, who have seen the highest increase of hate crimes, is the Jewish community.”

The equivalence point is a chemistry term you"ll encounter when you do a titration. However, it technically applies to any acid-base or neutralization reaction. Here"s its definition and a look at methods used to identify it.
The equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte solution. The moles of titrant (standard solution) equal the moles of the solution with unknown concentration. This is also known as the stoichiometric point because it is where the moles of acid are equal to the amount needed to neutralize the equivalent moles of base. Note this does not necessarily mean the acid to base ratio is 1:1. The ratio is determined by the balanced acid-base chemical equation.
The equivalence point is not the same as the endpoint of a titration. The endpoint refers to the point at which an indicator changes color. More often than not, the color change occurs after the equivalence point has already been reached. Using the endpoint to calculate equivalence naturally introduces error.
The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution.
In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. The acid to base ratio is not necessarily 1:1, but must be determined using the balanced chemical equation.
Methods of determining the equivalence point include color change, pH change, formation of a precipitate, change in conductivity, or temperature change.
Color Change - Some reactions naturally change color at the equivalence point. This may be seen in redox titration, particularly involving transition metals, where the oxidation states have different colors.
pH Indicator - A colored pH indicator may be used, which changes color according to pH. The indicator dye is added at the beginning of the titration. The color change at the endpoint is an approximation of the equivalence point.
Precipitation - If an insoluble precipitate forms as a result of the reaction, it can be used to determine the equivalence point. For example, the silver cation and chloride anion react to form silver chloride, which is insoluble in water. However, it can be difficult to determine precipitation because the particle size, color, and sedimentation rate may make it difficult to see.
Conductance - Ions affect the electrical conductivity of a solution, so when they react with each other, the conductivity changes. Conductance may be a difficult method to use, especially if other ions are present in the solution that can contribute to its conductivity. Conductance is used for some acid-base reactions.
Isothermal Calorimetry - The equivalence point may be determined by measuring the amount of heat that is produced or absorbed using a device called an isothermal titration calorimeter. This method is often used in titrations involving biochemical reactions, such as enzyme binding.
Spectroscopy- Spectroscopy can be used to find the equivalence point if the spectrum of the reactant, product, or titrant is known. This method is used to detect etching of semiconductors.
Thermometric Titrimetry- In thermometric titrimetry, the equivalence point is determined by measuring the rate of temperature change produced by a chemical reaction. In this case, the inflection point indicates the equivalence point of an exothermic or endothermic reaction.
Amperometry- In an ampometric titration, the equivalence point is seen as a change in the measured current. Amperometry is used when the excess titrant is able to be reduced. The method is useful, for example, when titrating a halide with Ag+ because it isn"t affected by precipitate formation.




 8613371530291
8613371530291