rongsheng jin lab free sample

Lam, K. H., Guo, Z., Krez, N., Matsui, T., Perry, K., Weisemann, J., Rummel, A., Bowen, M. E. & Jin, R. A viral-fusion-peptide-like molecular switch drives membrane insertion of botulinum neurotoxin A1. Nat Commun 9, 5367 (2018) doi: 10.1038/s41467-018-07789-4.
Chen, P., Tao, L., Liu, Z., Dong, M. & Jin, R. Structural insight into Wnt signaling inhibition by Clostridium difficile toxin B. FEBS J (2018) doi: 10.1111/febs.14681.
Chen, P., Tao, L., Wang, T., Zhang, J., He, A., Lam, K. H., Liu, Z., He, X., Perry, K., Dong, M*. & Jin, R*. Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B. Science 360, 664-669 (2018) (*corresponding authors) doi: 10.1126/science.aar1999. PMCID: PMC6231499
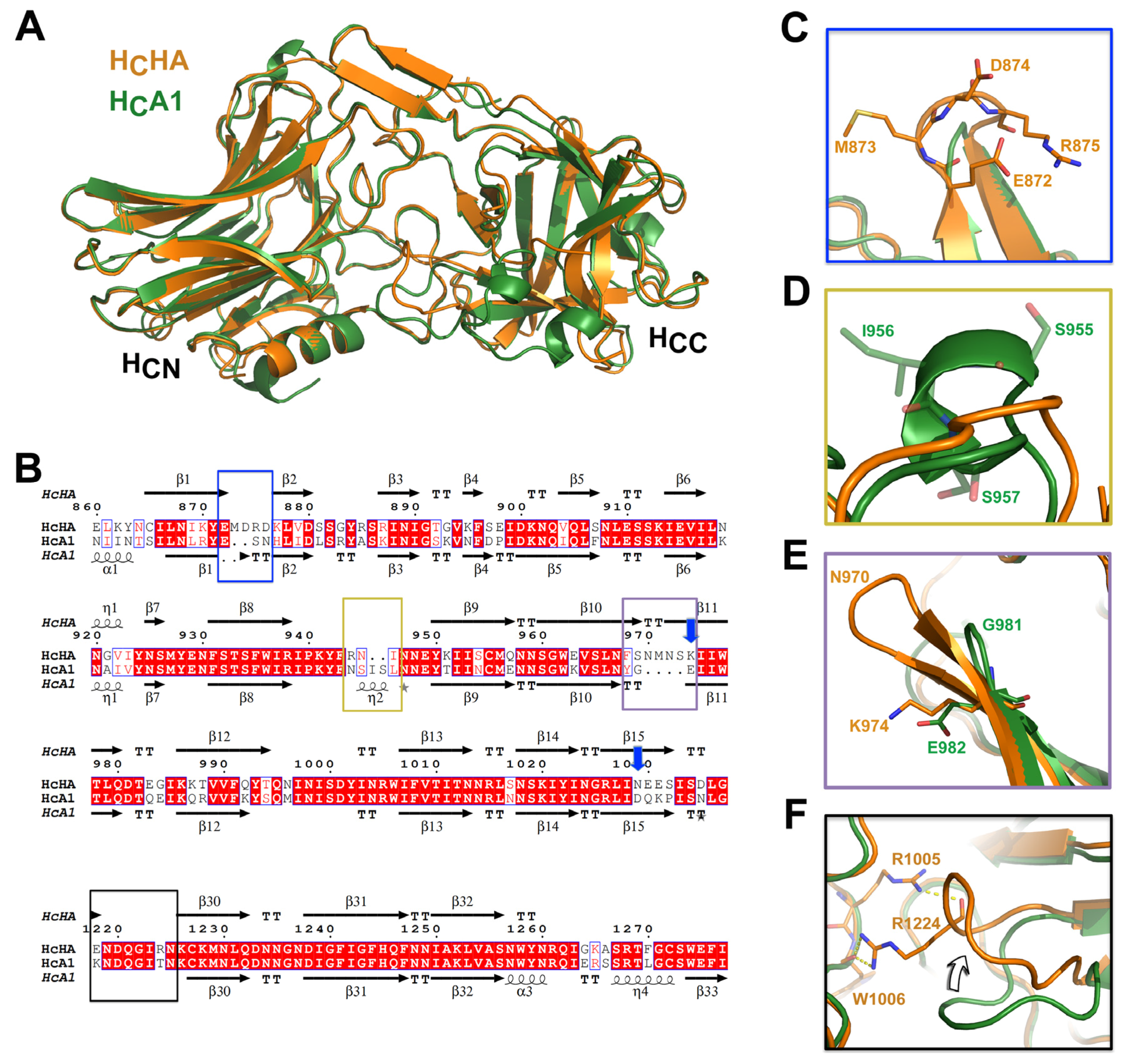
Lam, K. H., Sikorra, S., Weisemann, J., Maatsch, H., Perry, K., Rummel, A., Binz, T. & Jin, R. Structural and biochemical characterization of the protease domain of the mosaic botulinum neurotoxin type HA. Pathog Dis 76 (2018) doi: 10.1093/femspd/fty044. PMCID: PMC5961070
Silva, D. A., Stewart, L., Lam, K. H., Jin, R. & Baker, D. Structures and disulfide cross-linking of de novo designed therapeutic mini-proteins. FEBS J 285, 1783-1785 (2018) doi: 10.1111/febs.14394. PMCID: PMC6001749
Lam, K. H., Qi, R., Liu, S., Kroh, A., Yao, G., Perry, K., Rummel, A. & Jin, R. The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein. Toxicon 147, 19-26 (2018) doi: 10.1016/j.toxicon.2017.10.012. PMCID: PMC5902665
Chevalier, A., Silva, D.A., Rocklin, G.J., Hicks, D.R., Vergara, R., Murapa, P., Bernard, S.M., Zhang, L., Lam, K.H., Yao, G., Bahl, C.D., Miyashita, S.I., Goreshnik, I., Fuller, J.T., Koday, M.T., Jenkins, C.M., Colvin, T., Carter, L., Bohn, A., Bryan, C.M., Fernández-Velasco, D.A., Stewart, L., Dong, M., Huang, X., Jin, R., Wilson, I.A., Fuller, D.H. & Baker, D. Massively parallel de novo protein design for targeted therapeutics. Nature 550(7674):74-79 (2017) doi: 10.1038/nature23912. PMCID: PMC5802399
Yao, G., Lam, K.H., Weisemann, J., Peng, L., Krez, N., Perry, K., Shoemaker, C.B., Dong, M., Rummel, A. & Jin, R. A camelid single-domain antibody neutralizes botulinum neurotoxin A by blocking host receptor binding. Sci Rep. 7;7(1):7438. (2017) doi: 10.1038/s41598-017-07457-5. PMCID: PMC5547058
Yao, G., Lam, K.H., Perry, K., Weisemann, J., Rummel, A. & Jin, R. Crystal Structure of the Receptor-Binding Domain of Botulinum Neurotoxin Type HA, Also Known as Type FA or H. Toxins (Basel) 9, 93 (2017) doi: 10.3390/toxins9030093. PMCID: PMC5371848
Yao, G., Zhang, S., Mahrhold, S., Lam, K. H., Stern, D., Bagramyan, K., Perry, K., Kalkum, M., Rummel, A.*, Dong, M.* & Jin, R.* N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat Struct Mol Biol 23 (7):656-662 (2016) (*corresponding authors) doi: 10.1038/nsmb.3245. PMCID: PMC5033645
Lee, K., Lam, K. H., Kruel, A. M., Mahrhold, S., Perry, K., Cheng, L. W., Rummel, A. & Jin, R. Inhibiting oral intoxication of botulinum neurotoxin A complex by carbohydrate receptor mimics. Toxicon 107, 43-49 (2015) doi: 10.1016/j.toxicon.2015.08.003. PMCID: PMC4658216
Lam, K.H. & Jin, R. Architecture of the botulinum neurotoxin complex: a molecular machine for protection and delivery. Current Opinion in Structural Biology 31:89-95 (2015) doi: 10.1016/j.sbi.2015.03.013. PMCID: PMC4476938
Lam, K.H., Yao, G. & Jin, R. Diverse binding modes, same goal: The receptor recognition mechanism of botulinum neurotoxin. Progress in Biophysics and Molecular Biology 117(2-3):225-31 (2015) doi: 10.1016/j.pbiomolbio.2015.02.004. PMCID: PMC4417461
Lam, T.I., Stanker, L.H., Lee, K., Jin, R. & Cheng, L.W. Translocation of botulinum neurotoxin serotype A and associated proteins across the intestinal epithelia. Cellular Microbiology 17(8):1133-1143 (2015) doi: 10.1111/cmi.12424. PMCID: PMC4610714
Matsui, T.*, Gu, S., Lam, K.H., Carter, L.G., Rummel, A., Mathews, II. & Jin, R.* Structural Basis of the pH-Dependent Assembly of a Botulinum Neurotoxin Complex. J. Mol. Biol. 426(22):3773-3782 (2014) doi: 10.1016/j.jmb.2014.09.009. (*corresponding authors) PMCID: PMC4252799
Lee, K., Zhong, X., Gu, S., Kruel, A.M., Dorner, M.B., Perry, K., Rummel, A., Dong, M. & Jin, R. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science 344(6190):1405-1410 (2014) doi: 10.1126/science.1253823. PMCID: PMC4164303
Lee, K., Lam, K.H., Kruel, A.M., Perry, K., Rummel, A. and Jin, R. High-resolution crystal structure of HA33 of botulinum neurotoxin type B progenitor toxin complex. Biochem. Biophys. Res. Commun. 446(2):568-573 (2014) doi: 10.1016/j.bbrc.2014.03.008. PMCID: PMC4020412
Yao, Y., Lee, K., Gu, S., Lam, K.H. & Jin, R. Botulinum Neurotoxin A Complex Recognizes Host Carbohydrates through Its Hemagglutinin Component, Toxins (Basel) 6(2):624-635 (2014) doi: 10.3390/toxins6020624. PMCID: PMC3942755
Lee, K., Gu, S., Jin, L., Le, T.T.N., Cheng, L.W., Strotmeier, J., Kruel, A.M., Yao, G., Perry, K., Rummel, A.* & Jin, R.* Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLoS Pathog. 9(10): e1003690 (2013) doi:10.1371/journal.ppat.1003690. (*corresponding authors) PMCID: PMC3795040
Zong, Y. and Jin, R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 70(17):3077-88 (2013) doi: 10.1007/s00018-012-1209-9. PMCID: PMC4627850
Gu, S. and Jin, R. Assembly and function of the botulinum neurotoxin progenitor complex. Curr. Top. Microbiol. Immunol. 364:21-44 (2013) doi: 10.1007/978-3-642-33570-9_2. PMCID: PMC3875173
Gu, S., Rumpel, S., Zhou, J., Strotmeier, J., Bigalke, H., Perry, K., Shoemaker, C.B., Rummel, A. & Jin, R. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science 335(6071):977-81 (2012) doi: 10.1126/science.1214270. PMCID: PMC3545708
Zong, Y., Zhang, B., Gu, S., Lee, K., Zhou, J., Yao, G., Figueiredo, D., Perry, K., Mei, L.* & Jin, R.* Structural basis of neuron-specific regulation of postsynaptic differentiation. Gene & Development 26:247-258 (2012) doi: 10.1101/gad.180885.111. (*corresponding authors) PMCID: PMC3278892
Yao, G., Zong, Y., Gu, S., Zhou, J., Xu, H., Mathews, II. & Jin, R. Crystal structure of the glutamate receptor GluA1 amino-terminal domain. Biochem. J. 438(2):255-63 (2011) doi: 10.1042/BJ20110801. PMCID: PMC3296483
Strotmeier, J., Gu, S., Jutzi, S., Mahrhold, S., Zhou, J., Pich, A., Eichner, T., Bigalke, H., Rummel, A.*, Jin, R.* & Binz, T*. The biological activity of botulinum neurotoxin type C is dependent upon novel types of ganglioside binding sites. Mol. Microbiol. 81(1):143-56 (2011) doi: 10.1111/j.1365-2958.2011.07682.x. Epub 2011 Jun 2. (*corresponding authors)
Strotmeier, J., Lee, K., Völker, A.K., Mahrhold, S., Zong, Y., Zeiser, J., Zhou, J., Pich, A., Bigalke, H., Binz, T., Rummel, A.* & Jin, R.* Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 431(2):207-16 (2010) (*corresponding authors)
Jin, R.*, Singh, S.K., Gu, S., Furukawa, H., Sobolevsky, A.I., Zhou, J., Jin, Y. & Gouaux E.* Crystal structure and association behavior of the GluR2 amino-terminal domain. EMBO J. 28(12):1812-23 (2009) (*corresponding authors) PMCID: PMC2699365
Kumar, J., Schuck. P., Jin, R. & Mayer, M.L. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat. Struct. Mol. Biol. 16(6):631-8 (2009) PMCID: PMC2729365
Jin, R., Rummel, A., Binz, T. & Brunger, A.T. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 444:1092-5 (2006)
Jin, R., Clark, S., Weeks, A.M., Dudman, J.T., Gouaux, E. & Partin, K.M. Mechanism of positive allosteric modulators acting on AMPA receptors. J. Neurosci. 25(39):9027-36 (2005)
Jin, R., Junutula, J.R., Matern, H.T., Ervin, K.E., Scheller, R.H. & Brunger, A.T. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 24:2064-74 (2005)
Jin, R., Bank, T., Mayer, M. L., Traynelis, S. & Gouaux, E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 6(8):803-10 (2003)

Jahid S, Ortega JA, Vuong LM, Acquistapace IM, Hachey SJ, Flesher JL, La Serra MA, Brindani N, La Sala G, Manigrasso J, Arencibia JM, Bertozzi SM, Summa M, Bertorelli R, Armirotti A, Jin R, Liu Z, Chen CF, Edwards R, Hughes CCW, De Vivo M, Ganesan AK. PMID: 35385746; PMCID: PMC9127750.
Chen P, Zeng J, Liu Z, Thaker H, Wang S, Tian S, Zhang J, Tao L, Gutierrez CB, Xing L, Gerhard R, Huang L, Dong M, Jin R. PMID: 34145250; PMCID: PMC8213806.
Chen P, Lam KH, Liu Z, Mindlin FA, Chen B, Gutierrez CB, Huang L, Zhang Y, Hamza T, Feng H, Matsui T, Bowen ME, Perry K, Jin R. PMID: 31308519; PMCID: PMC6684407.

Wild type and mutated recombinant full-length activated BoNT/A1 were produced under biosafety level 2 containment (project number GAA A/Z 40654/3/123) recombinantly in K12 E. coli strain in Dr. Rummel’s lab6-tag were purified on Co2+-Talon matrix (Takara Bio Europe S.A.S., France) and eluted with 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 250 mM imidazole. For proteolytic activation, BoNT/A1 was incubated for 16 h at room temperature with 0.01 U bovine thrombin (Sigma-Aldrich Chemie GmbH, Germany) per µg of BoNT/A1. Subsequent gel filtration (Superdex-200 SEC; GE Healthcare, Germany) was performed in phosphate buffered saline (pH 7.4).
The purified ciA-D12 (S124C) was labeled with Alexa Fluor C2 647 maleimide (Molecular Probes) according to the manufacturer’s instructions. The labeled ciA-D12 was further purified by MonoQ ion-exchange chromatography in 10 mM Hepes (pH 8.0) and eluted with a NaCl gradient. The calculated dye to protein ratio was ~1 mole of dye per mole of ciA-D12. BoNT/A1i-ciA-D12 complex were prepared by mixing BoNT/A1i and ciA-D12 in 1:1.5 molar ratio and the complex was purified by size-exclusion chromatography using Superdex-200.
Liposomes containing 10% GT1b, 69% DOPC, 20% DOPS, 0.5% rhodamine-PE, and 0.5% biotin-PE were prepared by extrusion through a 100 nm pore membrane. To form proteoliposomes, 10 nM BoNT/A1i–D12* or oxidized BoNT/A1iDS–D12* was incubated with 0.5 mg/ml lipid at room temperature for 1 h at the pH indicated. The mixture was then diluted 1000-fold and incubated for 5 minutes in a passivated, quartz microscope chamber functionalized with streptavidin. The biotinylated liposomes were retained and unbound proteins are washed away by exhaustive rinsing with buffer. At the low densities needed for optical resolution of individual liposomes, we could observe sufficient liposomes for statistical analysis, while minimizing the probability that a diffraction-limited spot would contain multiple liposomes. Samples were imaged using a prism-based Total Internal Reflection Fluorescence (TIRF) microscope. Samples were first excited with a laser diode at 640 nm (Newport Corporation, Irvine, CA) to photobleach Alexa 647-labeled BoNT/A1i–D12* or BoNT/A1iDS-D12* molecules followed by excitation with a diode pumped solid state laser at 532 nm (Newport Corporation, Irvine, CA) to probe for Rhodamine-labeled liposomes. Emission from protein and lipids was separated using an Optosplit ratiometric image splitter (Cairn Research Ltd., Faversham UK) containing a 645 nm dichroic mirror, a 585/70 band pass filter for Rhodamine, and a 670/30 band pass filter for Alexa 647 (all Chroma, Bellows Falls, VT). The replicate images were relayed to a single iXon EMCCD camera (Andor Technologies, Belfast, UK) at a frame rate of 10 Hz. Data were processed in MATLAB to cross-correlate the replicate images and extract time traces for diffraction-limited spots with intensity above baseline. Single-molecule traces were hand selected based on the exhibition of single-step decays to baseline during 640 illumination and the appearance of Rhodamine emission during 532 illumination.
Zheng Liu, Conceptualization, Investigation, Visualization, Writing - original draft,1 Sicai Zhang, Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing,2 Peng Chen, Conceptualization, Methodology,1 Songhai Tian, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing,2 Ji Zeng, Investigation, Resources,2 Kay Perry, Investigation,3 Min Dong, Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing - original draft, Writing - review & editing,2 and Rongsheng Jin, Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing1
To understand how these structural differences exemplified by GTDVPI10463 and GTDM68 are related to the evolution of diverse TcdB variants, we carried out structure-based sequence analyses among all known TcdB variants available in DiffBase, which have been classified into 12 subfamilies (B1 to B12) (fig. S8) (18). We found that all these TcdB variants could be classified into two distinct groups based on their sequences in the GTPase-binding areas. Specifically, characteristic Rho-binding residues are conserved not only in other members of the same subfamily of TcdBVPI10463 (B1) but also in members of the B2, B5, B6, B9, B10, B11, and B12 subfamilies, while the R-Ras–binding residues are highly conserved in the B3 (including M68), B4, B7, and B8 subfamilies. Therefore, TcdB seems to have branched into two distinct groups during evolution that have developed two distinct sets of amino acids in their GTPase-binding areas to selectively recognize Rho or R-Ras. We propose to name them the RhoA group and R-Ras group, respectively, based on their preferred substrates, which cause two distinct types of cytopathic effects as reported in prior studies. We envision that these signature sequences in the GTD can be used to predict substrate specificity and pathogenicity of new C. difficile clinical strains that will emerge in the future.
The purified WT and mutant GTDs were transfected into HeLa cell via the Lipofectamine CRISPRMAX Cas9 Transfection Reagent from Thermo Fisher Scientific. The transfection was performed in coherence to the protocol provided by the manufacturer. HeLa cells at 5 × 104 per well were seeded into 24-well plates for overnight culture. For each well, we prepared solutions in tube 1 that contained 25 μl of Opti-MEM medium, 1250 ng of GTD, and 2.5 μl of Cas9 Plus Reagent and in tube 2 that contained 25 μl of Opti-MEM medium and 1.5 μl of CRISPRMAX reagent. We immediately added the solution from tube 1 to tube 2 and then mixed well. We incubated the complex for 10 min at room temperature then added all 50 μl of solution to the cells. The phase-contrast images of cells were taken at the indicated time (Olympus IX51; 10× objective). To test the full-length TcdB, HeLa cells were exposed to WT TcdBVPI10463 or TcdBVPI10463-α16/17M68 at indicated concentrations, and cells were imaged after overnight. Round-shaped and normal-shaped cells were counted manually. The percentage of round-shaped cells was analyzed using the OriginPro (OriginLab, v8.5) and Excel (Microsoft, 2007).
Funding: This work was partly supported by NIH grants R01AI125704, R21AI139690, and R21AI123920 to R.J.; R01NS080833 and R01AI132387 to M.D.; and R01AI139087 and R21 CA235533 to R.J. and M.D. M.D. holds the Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund. NE-CAT at the Advanced Photon Source (APS) is supported by a grant from the National Institute of General Medical Sciences (P30 GM124165), and the Eiger 16M detector on the 24-ID-E beamline is funded by an NIH-ORIP HEI grant (S10OD021527). Use of the APS, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under contract no. DE-AC02-06CH11357.
Data and materials availability: Atomic coordinates and structure factors of the Cdc42T35N–GTDVPI10463 and R-RasT61N–GTDM68 complexes have been deposited in the PDB under accession codes 7S0Y and 7S0Z, respectively. The x-ray diffraction data were processed with XDS as implemented in RAPD (https://github.com/RAPD/RAPD). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
34. Chen P., Lam K. H., Liu Z., Mindlin F. A., Chen B., Gutierrez C. B., Huang L., Zhang Y., Hamza T., Feng H., Matsui T., Bowen M. E., Perry K., Jin R.,
(A) Scattering profiles of BoNT/Ai at pH 8.0 (purple) and 6.0 (green). (i) Curve fitting of data at pH 8.0 with the crystal structure of free form BoNT/A. (ii) Curve fitting of data at pH 8.0 with the SAXS model determined by rigid body refinement. (iii) Superimposition of SAXS profiles at pH 8.0 and 6.0. Constant subtractions used in the rigid body refinements were taken into account. (iv) Curve fitting of data at pH 6.0 with the crystal structures of BoNT/A in the free (solid line) and the complex (dash line) forms. (v) Curve fitting of data at pH 6.0 with the SAXS model determined by rigid body refinement. All curve fittings were performed using the program CORAL [20]. (B) Kratky plots of the experimental profiles and theoretical curve of BoNT/Ai. (C) Pair distance distributions P(r) of BoNT/Ai at pH 8.0 and 6.0. Theoretical curves are shown as well. (D) The SAXS model of BoNT/Ai at pH 8.0 determined by rigid body refinement. The HC domain was refined as a rigid body (shown as brown tubes). Crystal structure of the free form BoNT/A is superimposed onto the LC–HN domain, with the HC domain labeled in purple tubes. The position of reconstructed loop is indicated by an arrow. (E) The SAXS model of BoNT/Ai at pH 6.0 with the HC domain labeled in cyan. (F) Comparison of the SAXS models between pH 8.0 and 6.0.
Citation:Lee K, Gu S, Jin L, Le TTN, Cheng LW, Strotmeier J, et al. (2013) Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLoS Pathog 9(10):
Structural information of HAs is available for serotypes C and D, such as the crystal structures of HA33 of serotype C (HA33-C) [12], [13], a complex composed of HA17 and HA33 of serotype D [14], and HA70 of serotype C (HA70-C) [15], [16]. However, BoNT/C and D rarely cause human botulism but are known to cause the syndrome in cattle, poultry, and wild birds. For BoNT/A, the major cause of human botulism, only the structure of HA33 (HA33-A), which displays an amino-acid identity of ∼38% to HA33-C and D, has been solved [17]. We have recently determined the crystal structure of the BoNT/A–NTNHA complex [5]. However, it remains largely unclear how the HAs assemble with one another and how they interact with BoNT and NTNHA.
Interacting residues are labeled in open-book views of the interfaces. (A) Interface between HA70 and HA17. (B) Interfaces between HA17 and the two HA33s are indicated by purple and blue. The HA33 residues involved in both interfaces are in green. (C) Interface between two HA33s attached to the same HA17. See Fig. S5 and S6 in Text S1 for stereo versions of the detailed interactions.
To determine the physiological relevance of these HA–glycan interactions during toxin absorption, we examined their ability to interfere with the HA complex-mediated disruption of Caco-2 TER. Lac, Gal, and IPTG markedly inhibited the TER reduction induced by the HA complex, and showed higher potencies when applied to the apical than to the basolateral compartment (Fig. 5A–B and Fig. S11A–D in Text S1). In contrast, α2,3- and α2,6-SiaLac, and to a lesser extent Neu5Ac, inhibited the decrease in TER only when applied apically, albeit more weakly than Lac (Fig. 5A–B and Fig. S11E–F in Text S1). We then examined the transport of the HA complex across the Caco-2 monolayer using a fluorescence-labeled HA complex (HA*) (Fig. 5C). Lac and IPTG efficiently inhibited the transport of HA* when it was applied to the apical or basolateral chamber. Blocking the transport of HA* via α2,3- and α2,6-SiaLac was more potent toward the basolateral compartment than toward the apical side. Neu5Ac at 50 mM did not inhibit transport of HA* from either side of the Caco-2 cell monolayer. These data are consistent with the ability of these carbohydrates to inhibit TER reduction induced by the HA complex. Collectively, these results suggest that the binding of HAs to Neu5Ac- and Gal-containing glycans on epithelial cells is essential for the transport of BoNT across the intestinal wall. Moreover, the carbohydrate receptors may play a more important role in the initial L-PTC absorption in the intestinal lumen, whereas other host receptors (e.g., E-cadherin) are involved once it gains access to the basolateral side.
(A, B) TER of Caco-2 monolayers was measured when Alexa-488-labeled HA complex (HA*) pre-incubated with Lac, IPTG, α2,3-SiaLac, or α2,6-SiaLac was applied to the apical (A; 58 nM) or basolateral (B; 17 nM) chambers. Values are means ± SD (n = 4–12). (C) HA* (with or without carbohydrates) or the Alexa-488-labeled HA33-DAFA complex (HA33-DAFA *) was applied to the apical (at 58 nM) or basolateral (at 17 nM) chamber. The fluorescence signals in both chambers were quantified after 24 hours and the amount of transported HA*/HA33-DAFA * was expressed as a percentage of the total HA*/HA33-DAFA * used. Values are means ± SD (n = 3–22).
The loose linkage between the M-PTC and the HA complex clearly suggests divided functions. We previously reported that the M-PTC"s compact structure protects BoNT against digestive enzymes and the extreme acidic environment of the GI tract [5], [23]. We now show that the HA complex is mainly responsible for BoNT absorption in the small intestine, through binding to specific host carbohydrate receptors. This new finding permitted the identification of IPTG as a prototypical oral inhibitor that extends survival following lethal oral BoNT/A intoxication of mice. Multivalent interactions involving nine binding sites for Neu5Ac- and Gal-containing glycans increase the overall avidity of binding between the L-PTC and glycans on the epithelial cell surface, and thus compensate for the modest glycan-binding affinities at individual binding sites (Fig. 6C). Similarly, the potency of carbohydrate receptor mimics could be improved by optimizing the HA–glycan interactions as revealed here or by introducing new HA–inhibitor interactions at individual binding sites based on rational design, as well as by designing multivalent inhibitors. Although such inhibitors cannot be used to treat fully developed food-borne botulism, they could provide temporary protection upon pre-treatment and could also be useful for cases of intestinal colonization with C. botulinum spores such as in cases of infant or adult intestinal botulism. Our results also suggest that the L-PTC could be exploited for alternative applications. For example, protein-based therapeutics could be coupled to the modified non-toxic L-PTC to allow oral delivery by improving drug stability, absorption efficiency, and bioavailability.
The purified HA70 was labeled with Alexa Fluor® 488 carboxylic acid, succinimidyl ester (Life Technologies) according to the manufacturer"s instructions. The labeled HA70 was further purified by Superdex 200 chromatography in 20 mM Tris (pH 8.0) and 50 mM NaCl. The calculated dye to protein ratio was ∼2 moles of dye per mole of monomeric HA70.
The HA17–HA33, the HA70–HA17, and the HA70D3–HA17 complexes were produced by co-expression and co-purification as described above. To assemble the mini-HA complex (HA70D3–HA17–HA33), the purified HA33 and the HA70D3–HA17 complex were mixed at a molar ratio of ∼2.5∶1 and incubated at 4°C overnight. The excess HA33 was removed by Superdex 200 chromatography with 20 mM Tris (pH 7.6) and 50 mM NaCl. The fully assembled HA complex was reconstituted by mixing the purified HA70 and the HA17–HA33 complex at a molar ratio of ∼1∶1.3. The mixture was incubated at 4°C overnight and the excess HA17–HA33 complex was removed from the mature HA complex by Superdex 200 chromatography with 20 mM Tris (pH 7.6) and 50 mM NaCl. The fluorescence-labeled HA complex was prepared with Alexa Fluor® 488-labeled HA70 and unlabeled HA17–HA33 complex (HA*) or HA17–HA33DAFA complex (HA33DAFA*) using a similar protocol.
Sedimentation equilibrium (SE) experiments were performed in a ProteomeLab XL-I (BeckmanCoulter) analytical ultracentrifuge. Purified HA samples were dialyzed extensively against a buffer containing 50 mM Tris (pH 7.6) and various NaCl concentrations, or 50 mM citric acid (pH 2.3) and various NaCl concentrations. Protein samples at concentrations of 0.4, 0.2, and 0.1 unit of OD280 were loaded in 6-channel equilibrium cells and centrifuged at 20°C in an An-50 Ti 8-place rotor at the first speed indicated until equilibrium was achieved and thereafter at the second speed. HA33 was analyzed at rotor speeds of 19,000 and 22,000 rpm. The HA17–HA33 and the HA70D3–HA17–HA33 complexes were analyzed at 12,000 and 14,000 rpm. The HA70–HA17 and the HA70–HA17–HA33 complexes were run at speeds of 6,000 and 8,000 rpm. For each sample, data sets for the two different speeds were analyzed independently using HeteroAnalysis software (by J.L. Cole and J.W. Lary, University of Connecticut). Three independent experiments were performed for each sample.
The L-PTC of BoNT/A was obtained from List Biological Laboratories, Inc. (Campbell, California) and Miprolab GmbH (Göttingen, Germany). The recombinant HA complex was reconstituted in vitro as described above. Negatively stained EM specimens were prepared following a previously described protocol [54]. Briefly, 3 µl of the L-PTC (∼0.02 mg/ml in 20 mM MES, pH 6.2, and 100 mM NaCl) or the HA complex (∼0.01 mg/ml in 20 mM Tris, pH 7.6, and 50 mM NaCl) was placed on a freshly glow-discharged carbon-coated EM grid, blotted with filter paper after 40 seconds, washed with two drops of deionized water, and then stained with two drops of freshly prepared 1% uranyl formate, which also served to fix the proteins.
Carbohydrate inhibition assays: Lac, Gal, IPTG, Neu5Ac, α2,6- and α2,3-SiaLac were dissolved in IMDM, sterile filtered and stored at −20°C. Neu5Ac stock solution was adjusted to pH 7.4. The wild type HA complex (HA wt), fluorescence-labeled HA complex (HA*), or the HA70-TPRA complex were pre-incubated with the corresponding carbohydrate over night at 4°C in IMDM and diluted to the final concentration with IMDM prior to administration. The TER upon administration of each carbohydrate in the highest concentrations used was checked in the absence of HA and was virtually identical to that of the control without sugars.
Transport measurement: For paracellular transport studies, filters were incubated in IMDM added to the apical (0.5 ml) and basolateral (1.5 ml) reservoir. As marker substance Alexa Fluor® 488 labeled HA* or HA33-DAFA* was administered to the apical or basolateral reservoirs at final concentrations of 58 nM and 17 nM, respectively. After 24 hour of incubation, 200 µl of samples were taken from the apical and the basolateral reservoir. The marker substance was measured in a BioTek Synergy 4 fluorescence spectrophotometer at 495 nm excitation and 519 nm emission wavelengths.
4.Cheng LW, Onisko B, Johnson EA, Reader JR, Griffey SM, et al. (2008) Effects of purification on the bioavailability of botulinum neurotoxin type A. Toxicology 249: 123–129.
20.Inoue K, Fujinaga Y, Watanabe T, Ohyama T, Takeshi K, et al. (1996) Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun 64: 1589–1594.
25.Fujinaga Y, Inoue K, Nomura T, Sasaki J, Marvaud JC, et al. (2000) Identification and characterization of functional subunits of Clostridium botulinum type A progenitor toxin involved in binding to intestinal microvilli and erythrocytes. FEBS Lett 467: 179–183.
26.Inoue K, Fujinaga Y, Honke K, Arimitsu H, Mahmut N, et al. (2001) Clostridium botulinum type A haemagglutinin-positive progenitor toxin (HA(+)-PTX) binds to oligosaccharides containing Gal beta1-4GlcNAc through one subcomponent of haemagglutinin (HA1). Microbiology 147: 811–819.
27.Sugawara Y, Matsumura T, Takegahara Y, Jin Y, Tsukasaki Y, et al. (2010) Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J Cell Biol 189: 691–700.
28.Jin Y, Takegahara Y, Sugawara Y, Matsumura T, Fujinaga Y (2009) Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins - differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology 155: 35–45.
29.Matsumura T, Jin Y, Kabumoto Y, Takegahara Y, Oguma K, et al. (2008) The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol 10: 355–364.
32.Fujita R, Fujinaga Y, Inoue K, Nakajima H, Kumon H, et al. (1995) Molecular characterization of two forms of nontoxic-nonhemagglutinin components of Clostridium botulinum type A progenitor toxins. FEBS Lett 376: 41–44.
33.Ohyama T, Watanabe T, Fujinaga Y, Inoue K, Sunagawa H, et al. (1995) Characterization of nontoxic-nonhemagglutinin component of the two types of progenitor toxin (M and L) produced by Clostridium botulinum type D CB-16. Microbiol Immunol 39: 457–465.

In foodborne botulism, BoNT must cross intestinal epithelial barriers to enter the blood stream and reach target neurons. The mechanism of how the large BoNT holotoxin or its multi-subunit complexes traverse the polarized epithelial monolayer is not fully understood. The three-dimensional structure of the BoNT/A complex has recently been elucidated (Gu etal., 2012; Lee etal., 2013). Carbohydrate binding sites have been identified and recently shown to be involved in binding to intestinal epithelia. BoNT holotoxins are known to cross the intestinal epithelium from the apical side and relocate to the basolateral side via transcytosis (Maksymowych and Simpson, 1998; 2004; Ahsan etal., 2005; Fujinaga etal., 2009; Fujinaga, 2010). Large BoNT complexes have been shown to increase oral toxicity in mice by about 20 times over that of purified holotoxin. Toxin complex size is directly proportional to oral toxicity: the larger the complex, the greater the oral toxicity (Ohishi etal., 1977; Sugii etal., 1977; Chen etal., 1998). Studies by others suggest an active role for the NAPs, such as HA33 binding to surface receptors followed by internalization and transcytosis. HA33 was shown to disrupt epithelial tight junctions from the basolateral side, promoting rapid toxin complex passage via a paracellular mechanism (Fujinaga etal., 2009; Jin etal, 2009).
There are at least two proposed mechanisms of BoNT translocation. The first purports that NAPs only protect BoNTs from the low pH and degradative effects of intestinal juices and play no role in holotoxin uptake. In this model, BoNT holotoxin alone is able to traverse the intestinal and lung epithelium and reach the bloodstream intact (Maksymowych etal., 1999; Ahsan etal., 2005; Al-Saleem etal., 2012; Couesnon etal., 2012; Simpson, 2013). In a second model, NAPs play a direct function in binding intestinal receptors, disrupting tight junctions in the intestinal epithelial cell barrier, and promoting the paracellular transport of BoNTs following initial transcytosis (Matsumura etal., 2008; Jin etal., 2009; Sugawara etal., 2010). However, most of the evidence supporting either hypothesis is derived from in vitro cultured intestinal cell models and limited ex vivo studies that do not provide a complete picture of how toxin complexes translocate across the intestinal epithelia.
Purified BoNT/A complex is composed of the 150 kDa holotoxin (about 30% of total weight), the NTNH (140 kDa) and the HA complex (HA-C) of about 470 kDa which includes HA70, HA33 and HA17 (Supporting Information Fig. S1) (Cheng etal., 2008). To compare the internalization of each component of the toxin complex, we first used Caco-2, human colon adenocarcinoma cells as the in vitro model. Cells were grown in tissue culture media at 37°C at neutral pH and were treated with 50 ng/ well of the BoNT/A complex (AC), 15ng/well of the BoNT/A holotoxin (AHT) or with 20 ng/well of HA-C for 4 h. Immunofluorescence staining with the rabbit anti-BoNT/A antibodies showed the presence of BoNT/A complex and holotoxin throughout the depth of Caco-2 cells after 4 h of incubation, indicating internalization of toxins from the surface of cells to the cytosol (Fig. 1, Supporting Information Figs S2 and S3). Labelled mAb against HA70 revealed internalization of HA70 in both the BoNT/A complex and the HA-C after 4 h. Probing with the NTNH monoclonal antibody showed internalization of NTNH only in the BoNT/A complex, as NTNH is not present in the HA-C. The signal intensities for BoNT/A, HA70 and NTNH in the immunofluorescence images were quantified with ImageJ for comparison (Fig. 1B-D). A 63x magnification view of Caco-2 cells treated with either BoNT/A holotoxin or complex showed the presence of BoNT/A labelled vesicles indicative of cellular transcytosis (Supporting Information Fig. S4).
A. Caco-2 cells were treated with media (control) or with BoNT/A complex (AC), holotoxin (AHT) or recombinant HA complex (HA-C) for 4 h at 37°C. Cells were stained with of Alexa-488 labelled antibodies against BoNT/A, HA70 and NTNH. The fluorescence signals for BoNT/A, HA70 and NTNH were quantitated by determining mean fluorescence from Caco-2 cells from three randomly chosen optical fields from each of four to five coverslips per experiment using ImageJ software (B-D). Values represent means of four independent experiments ± SEM.
statistically significant until 6 h after adding the toxin. There was minimal background HA70 or NTNH immuno-fluorescence signal as expected. Furthermore, we confirmed that the HA-C could bind Caco-2 cells independently of BoNT/A holotoxin. Anti-HA70 immunofluores-cence from cells treated with the HA-C showed a similar binding and internalization pattern as that of the HA70 signal associated with the BoNT/A complex (Fig. 2C). When BoNT/A holotoxin was added to Caco-2 cells along with the recombinant HA-C, binding and internalization of BoNT/A resembled that of holotoxin alone. Thus, in the absence of a physical interaction between the holotoxin and HA-C through NTNH, the HA-C did not enhance holotoxin entry (Fig. 2D). The internalization of BoNT and NAPs into the Caco-2 cells was visualized in Z stacked images of the labelled cells (Supporting Information Fig. S2). Caco-2 cells were labelled with fluorescence-
labelled BoNT/A throughout the depth of cells and showed the typical ringed labelling where there is nuclei exclusion and presence of toxin-labelled vesicles (Supporting Information Figs S2 and S4). This significant difference in the temporal pattern of binding indicates that NAPs reduced the amount of time needed to cross epithelia perhaps by actively promoting receptor binding and presumably carrying the holotoxin inside.
HA33, a component of the HA-C, was previously shown to disrupt membrane tight junctions (Fujinaga etal., 2009; Jin etal., 2009). However, these studies utilized unusual growth conditions such as low temperatures as well as very large amounts of toxins. To determine the effect of
Little is known regarding the fate of BoNT once ingested. Previous studies have used ex vivo models or ligated intestines to examine the effects of exposure to recombinant BoNT complexes or fragments of BoNTs (Couesnon etal., 2012; Lee etal., 2014). Here we used the mouse oral model of intoxication to study the translocation of the native BoNT/A holotoxin, the BoNT/A complex and the recombinant HA-C. Passages of BoNT and NAPs through the small intestine were tracked over 24 h (Figs 4 and 5 and Supporting Information Fig. S5). Similar to the in vitro findings, immunofluorescence-labelled intestinal villi from mice fed with BoNT/A complex showed initial binding of BoNT/A starting at 2 h and peaked at 6 h. However, by 8 h, there is a decrease in immunofluorescence signal suggesting BoNT/A
Fig. 4. Binding and translocation of BoNT/A, HA70 and NTNH through the mouse intestinal villi over time. Mice were given either BoNT/A complex (A), BoNT/A holotoxin (B), HA complex (C), or BoNT/A holotoxin and HA complex (D) by oral gavage. Segments of the upper small intestine were harvested over 24 h and labelled with a rabbit polyclonal antibody against BoNT/A, and mAbs against HA70 and NTNH. The immunofluorescence intensity was quantified by fluorescent thresholding using ImageJ software. Values represent mean signal intensities ± SEM (n = 4). Time 0 h represents control animals not treated with toxin or NAPs.
Fig. 5. Immunofluorescence tracking of the BoNT/A complex, BoNT/A holotoxin and HA complex entry through the mouse small intestinal villi. Representative confocal images of small intestinal villi sections harvested at 2, 4, 6, 8 and 24 h after oral gavage with BoNT/A complex (AC), BoNT/A holotoxin (AHT), HA complex (HA-C) or BoNT/A holotoxin with HA complex. Tissues were labelled with anti-BoNT/A (green) and HA70 (red). DAPI (blue) provided nuclear counterstaining. Images were shown at 40x magnification. (n = 4 mice per time point).
holotoxin clearance into the lymph or blood (Figs 4A and 5A). At the intestinal crypts, we found that BoNT/A complex started to appear at the crypts from 2 to 8 h and a decrease in toxin staining by 8 h (Supporting Information Fig. S6). The immunofluorescence signals for HA70 and NTNH showed initial binding that occurred at 2 h and plateaued after 6 h (Fig. 5A and Supporting Information Fig. S7), much like BoNT/A in the complex. Immunofluorescence-labelled intestinal villi from BoNT/A holotoxin fed with mice showed a significant binding that occurred at 4 h and peaked at 8 h. HA70 and NTNH were absent from BoNT/A holotoxin mice, as expected (Figs 4B and 5B). We also evaluated intestinal crypts from these tissues. Immunofluorescence images showed that holotoxin appeared in the crypts from 4 to 8 h with a decrease in immunostaining at 24 h (Supporting Information Fig. S6). Treatment of mice with the recombinant HA-C showed similar HA70 immunofluorescence pattern to BoNT/A complex, binding at 2 h and plateaued after 6 h with a longer sustained staining of HA70 at 24 h,
What we know thus far from in vivo and ex vivo assays is that BoNT itself can transcytose across the intestinal epithelium (Fujinaga, 2010; Couesnon etal., 2012; Yao etal., 2014). Yet, there is a body of indirect and direct evidence which argues that the HA proteins in NAPs can interact with the intestinal epithelia. In one hypothesis, Fujinaga etal. proposed a three-step mechanism: (i) transcytosis, (ii) intestinal barrier disruption and (iii) entry of toxin through damaged epithelia (Fujinaga, 2010). The HAs bind mainly on the basolateral surface inducing loss of the paracellular barrier or cause cell damage, allowing toxin to move into the cells. HA33 was shown to facilitate transport of BoNT/D across epithelium and treatment with antibodies against HA70 and HA33 as well as incubation with sialic acid reduced cell binding and transport (Hasegawa etal., 2007). Recently, HA proteins were shown to interact with intestinal saccharides in a sialic acid or galactose-dependent process. Binding and transport were inhibited with anti-HA33 antibodies or the addition of saccharides (Ito etal., 2011). Although BoNT holotoxins alone can enter intestinal epithelial cells, NAPs play a role to facilitate its internalization.
What role do NAPs play in translocation? Previous data suggested that NAPs, specifically HA33, can disrupt tight junctions, compromising the epithelial cell barrier and allowing BoNT complex to enter through a paracellular pathway (Fujinaga, 2010). The HA-C was also shown to bind E-cadherin, a cell adhesion protein that likely plays a major role in toxin cell surface binding (Sugawara etal., 2010; Lee etal., 2014). The HA-C was observed to disrupt membrane integrity (measured by TER) quickly after toxin or HA addition and much faster after addition of toxins or HA to the basolateral side. These data combined with the location of E-cadherin in the intercellular space suggested that the paracellular translocation pathway was the likely route for toxin complex entry (Lee etal., 2014). In contrast, our results from measuring TER of polarized
Our results also contrast with ex vivo studies where upper intestinal sections were treated with fluorescent-labelled BoNT/A heavy chain at 4°C for 30-120 min (Couesnon etal., 2012). These studies showed that the entry site for the heavy chain domain of BoNT/A is predominantly located in the intestinal crypt cells, and then targets the submucosa and musculosa neurons. Yao etal. also showed in ex vivo studies that NAPs bind to the small intestines and that the binding was likely through the interaction between HA70 and HA33 with host carbohydrates (Yao etal., 2014). Using this model, they did not observe any holotoxin binding but that was likely because of the short exposure time (30 min). Toxin entry could be interrupted with the addition of receptor-mimicking saccharides suggesting an HA-mediated
Some BoNT subtypes (such as BoNT/A2 and BoNT/A3) and BoNT/E toxin complexes contain alternate, non-HA-type NAPs such as OrfXs, p21 or p47 (Hill etal., 2007). How these NAPs contribute to toxicity differences, and how well mouse oral bioavailability data translate to human oral intoxication remain to be explored. A better understanding of how large toxin complexes traverse the intestinal epithelia could lead to a better understanding of toxin-mediated pathogenesis. Because BoNTs are also currently explored as vehicles for neuronal oral drug delivery, this could lead to the design and delivery of orally delivered drugs for a multitude of diseases.
Botulinum neurotoxin serotype A holotoxin, BoNT/A complex and rabbit polyclonal antibodies against BoNT/A were purchased from Metabiologics. The median lethal dose of BoNT/A holotoxin and complex was estimated at 0.42 ng kg-1 or about 8 pg/mouse by intraperitoneal injection and 27 ig kg-1 or about 0.5 ig/mouse for BoNT/A complex via oral gavage (Cheng et al., 2008). Caco-2, a human epithelial colon adenocarcinoma cell line, was purchased from the American Type Culture Collection. Chemicals and reagents were purchased from Sigma-Aldrich and tissue culture supplies were from Life Technologies. Monoclonal antibodies (mAbs): a mAb against the NTNH complex protein, referred to as NTNH 84-27-7-5, was generated following immunization with a recombinant protein corresponding to aa 616-1193 of the NTNH molecule and prepared in Phosphate Buffered Saline (PBS) by the Stanker laboratory (unpublished results); the HA70 specific mAb, NAP80-7-2-1 (Stanker etal., 2013). The recombinant HA-C was obtained from the Jin lab and was prepared as described in Lee etal. 2013 (Gu etal., 2012; Lee etal., 2013).
Double immunostaining was performed with either confluent Caco-2 monolayers on glass coverslips or small intestinal cryosections that were rinsed twice with PBS and permeabilized with 1% triton X-100 in PBS for 30 min. Samples were incubated with blocking solution (2% goat serum, 0.2% Triton X-100 and 0.1% Bovine Serum Albumin) for 1 h, and then incubated with 1:250 blocking buffer diluted solutions of a polyclonal rabbit antibody against BoNT/A (2 mg ml-1 of stock), and mAbs against either HA70 (1.6mgml-1 of stock) or NTNH (5.5 mg ml-1 of stock). After washing three times, cells or tissue were incubated with Alexa Fluor 488 to mouse IgG or Alexa Fluor 568 to rabbit IgG (1:500 dilution; Life Technologies) and mounted onto glass slides using the hard-set DAPI mounting medium (Vector Laboratories). Florescence signals were visualized with a Leica Microsystems confocal microscope (Leica TCS SP5) with the appropriate filter sets. For analysis of BoNT/A and HA70 inter-nalization into the intestinal epithelial cells, total fluorescence intensity was quantified in at least 81 optical fields taken from three independent experiments. For quantification, the mean fluorescence was measured in three randomly selected non-overlapping 40x fields with each containing approximately 100150 Caco-2 cells. Similarly, the mean fluorescence of the small intestinal tissue sections was measured in four separate 40x fields per animal in each time point. Raw fluorescence was then normalized to control levels and the percent increase in fluorescence was calculated from these normalized values. Negative controls were prepared by omitting the primary antibodies.
Mice were administered 0.6, 2 or 0.8 ig/mouse, respectively, of phosphate gelatin (0.028 M sodium phosphate, pH 6.2 and 0.2% gelatin) diluted BoNT/A holotoxin, complex and recombinant HA70 complex by gavage with popper feeding needles directly into the vicinity of mouse stomachs. At 2, 3, 4, 6, 7, 8 and 24 h post intoxication, three mice per time point were euthanized and perfusion-fixed with normal saline followed by 4% PFA. Control mice were orally treated with phosphate gelatin for 6 h. Segments of the small intestine were immersed in 4% PFA overnight, cryoprotected with 20% sucrose for 2 days, embedded in OCT embedding medium (Tissue-Tek, Miles Laboratories) and stored at -80°C. Cryosections (5 |m) were cut with a cryostat microtome and mounted onto SuperFrost glass slides (Fisher Scientific).
Cheng, L.W., Onisko, B., Johnson, E.A., Reader, J.R., Griffey, S.M., Larson, A.E., etal. (2008) Effects of purification on the bioavailability of botulinum neurotoxin type A. Toxicology 249: 123-129.
Fujinaga, Y., Matsumura, T., Jin, Y., Takegahara, Y., and Sugawara, Y. (2009) A novel function of botulinum toxin-associated proteins: HA proteins disrupt intestinal epithelial barrier to increase toxin absorption. Toxicon 54: 583586.
Inoue, K., Fujinaga, Y., Watanabe, T., Ohyama, T., Takeshi, K., Moriishi, K., etal. (1996) Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun 64: 1589-1594.
Jin, Y., Takegahara, Y., Sugawara, Y., Matsumura, T., and Fujinaga, Y. (2009) Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins - differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology 155: 35-45.
Lee, K., Gu, S., Jin, L., Le, T.T., Cheng, L.W., Strotmeier, J., etal. (2013) Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog 9: e1003690.
Matsumura, T., Jin, Y., Kabumoto, Y., Takegahara, Y., Oguma, K., Lencer, W.I., and Fujinaga, Y. (2008) The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol 10: 355-364.
Sugawara, Y., Matsumura, T., Takegahara, Y., Jin, Y., Tsukasaki, Y., Takeichi, M., and Fujinaga, Y. (2010) Botuli-num hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J Cell Biol 189: 691700.
Yao, G., Lee, K., Gu, S., Lam, K.H., and Jin, R. (2014) Botulinum neurotoxin A complex recognizes host carbohydrates through its hemagglutinin component. Toxins 6: 624-635.
Fig. S3. Internalization of BoNT/A holotoxin, BoNT/A complex into Caco-2 cells. (A) Caco-2 cells were treated with media (control) or with BoNT/A complex (AC), holotoxin (AHT) for 4 h at 37°C. Cells were stained with of Alexa-488 labelled antibodies against BoNT/A (green), phalloidin (red) and DAPI (blue). Cells were viewed under 40x magnification. (B) Zoomed-in view of Caco-2 cells treated with BoNT/A complex. (C) Zoomed-in view of Caco-2 cells treated with BoNT/A holotoxin. Fig. S4. BoNT/A staining in Caco-2 cells treated with complex (AC) and holotoxin (AHT). Cells were stained with rabbit anti-BoNT/A followed with goat anti-rabbit Alexa 488 (green) and DAPI (blue) and viewed under 63x magnification. Fig. S5. Translocation of BoNT/A complex, BoNT/A holotoxin and HA complex through the mouse intestinal villi. Representative confocal images of frozen sections of small intestinal villi illustrate the binding and internalization of BoNT/A (green) and HA70 (red) through the mouse intestinal epithelia. DAPI (blue) provides nuclear counterstaining. Samples were imaged at 40x, n = 4 mice per time point.

abstract = {Mixed-chirality peptide macrocycles such as cyclosporine are among the most potent therapeutics identified to date, but there is currently no way to systematically search the structural space spanned by such compounds. Natural proteins do not provide a useful guide: Peptide macrocycles lack regular secondary structures and hydrophobic cores, and can contain local structures not accessible with L-amino acids. Here, we enumerate the stable structures that can be adopted by macrocyclic peptides composed of L- and D-amino acids by near-exhaustive backbone sampling followed by sequence design and energy landscape calculations. We identify more than 200 designs predicted to fold into single stable structures, many times more than the number of currently available unbound peptide macrocycle structures. Nuclear magnetic resonance structures of 9 of 12 designed 7- to 10-residue macrocycles, and three 11- to 14-residue bicyclic designs, are close to the computational models. Our results provide a nearly complete coverage of the rich space of structures possible for short peptide macrocycles and vastly increase the available starting scaffolds for both rational drug design and library selection methods.},
Mixed-chirality peptide macrocycles such as cyclosporine are among the most potent therapeutics identified to date, but there is currently no way to systematically search the structural space spanned by such compounds. Natural proteins do not provide a useful guide: Peptide macrocycles lack regular secondary structures and hydrophobic cores, and can contain local structures not accessible with L-amino acids. Here, we enumerate the stable structures that can be adopted by macrocyclic peptides composed of L- and D-amino acids by near-exhaustive backbone sampling followed by sequence design and energy landscape calculations. We identify more than 200 designs predicted to fold into single stable structures, many times more than the number of currently available unbound peptide macrocycle structures. Nuclear magnetic resonance structures of 9 of 12 designed 7- to 10-residue macrocycles, and three 11- to 14-residue bicyclic designs, are close to the computational models. Our results provide a nearly complete coverage of the rich space of structures possible for short peptide macrocycles and vastly increase the available starting scaffolds for both rational drug design and library selection methods.
Aaron Chevalier*, Daniel-Adriano Silva*, Gabriel J. Rocklin*, Derrick R. Hicks, Renan Vergara, Patience Murapa, Steffen M. Bernard, Lu Zhang, Kwok-Ho Lam, Guorui Yao, Christopher D. Bahl, Shin-Ichiro Miyashita, Inna Goreshnik, James T. Fuller andMerika T. Koday, Cody M. Jenkins, Tom Colvin, Lauren Carter, Alan Bohn, Cassie M. Bryan, D. Alejandro Fernández-Velasco, Lance Stewart, Min Dong, Xuhui Huang, Rongsheng Jin, Ian A. Wilson, Deborah H. Fuller, David Baker
author = {Aaron Chevalier* and Daniel-Adriano Silva* and Gabriel J. Rocklin* and Derrick R. Hicks and Renan Vergara and Patience Murapa and Steffen M. Bernard and Lu Zhang and Kwok-Ho Lam and Guorui Yao and Christopher D. Bahl and Shin-Ichiro Miyashita and Inna Goreshnik and James T. Fuller andMerika T. Koday and Cody M. Jenkins and Tom Colvin and Lauren Carter and Alan Bohn and Cassie M. Bryan and D. Alejandro Fernández-Velasco and Lance Stewart and Min Dong and Xuhui Huang and Rongsheng Jin and Ian A. Wilson and Deborah H. Fuller and David Baker},
Marcos, Enrique*, Basanta, Benjamin*, Chidyausiku, Tamuka M., Tang, Yuefeng, Oberdorfer, Gustav, Liu, Gaohua, Swapna, G. V. T., Guan, Rongjin, Silva, Daniel-Adriano, Dou, Jiayi, Pereira, Jose Henrique, Xiao, Rong, Sankaran, Banumathi, Zwart, Peter H., Montelione, Gaetano T., Baker, David
author = {Marcos, Enrique* and Basanta, Benjamin* and Chidyausiku, Tamuka M. and Tang, Yuefeng and Oberdorfer, Gustav and Liu, Gaohua and Swapna, G. V. T. and Guan, Rongjin and Silva, Daniel-Adriano and Dou, Jiayi and Pereira, Jose Henrique and Xiao, Rong and Sankaran, Banumathi and Zwart, Peter H. and Montelione, Gaetano T. and Baker, David},
abstract = {Nature provides many examples of self- and co-assembling protein-based molecular machines, including icosahedral protein cages that serve as scaffolds, enzymes, and compartments for essential biochemical reactions and icosahedral virus capsids, which encapsidate and protect viral genomes and mediate entry into host cells. Inspired by these natural materials, we report the computational design and experimental characterization of co-assembling, two-component, 120-subunit icosahedral protein nanostructures with molecular weights (1.8 to 2.8 megadaltons) and dimensions (24 to 40 nanometers in diameter) comparable to those of small viral capsids. Electron microscopy, small-angle x-ray scattering, and x-ray crystallography show that 10 designs spanning three distinct icosahedral architectures form materials closely matching the design models. In vitro assembly of icosahedral complexes from independently purified components occurs rapidly, at rates comparable to those of viral capsids, and enables controlled packaging of molecular cargo through charge complementarity. The ability to design megadalton-scale materials with atomic-level accuracy and controllable assembly opens the door to a new generation of genetically programmable protein-based molecular machines.},
Nature provides many examples of self- and co-assembling protein-based molecular machines, including icosahedral protein cages that serve as scaffolds, enzymes, and compartments for essential biochemical reactions and icosahedral virus capsids, which encapsidate and protect viral genomes and mediate entry into host cells. Inspired by these natural materials, we report the computational design and experimental characterization of co-assembling, two-component, 120-subunit icosahedral protein nanostructures with molecular weights (1.8 to 2.8 megadaltons) and dimensions (24 to 40 nanometers in diameter) comparable to those of small viral capsids. Electron microscopy, small-angle x-ray scattering, and x-ray crystallography show that 10 designs spanning three distinct icosahedral architectures form materials closely matching the design models. In vitro assembly of icosahedral complexes from independently purified components occurs rapidly, at rates comparable to those of viral capsids, and enables controlled packaging of molecular cargo through charge complementarity. The ability to design megadalton-scale materials with atomic-level accuracy and controllable assembly opens the door to a new generation of genetically programmable protein-based molecular machines.
This work was partly supported by National Institute of Allergy and Infectious Diseases (NIAID) grants R01AI139087, R01AI158503, and R21AI156092 to RJ. NE-CAT at the Advanced Photon Source (APS) is supported by a grant from the National Institute of General Medical Sciences (P30 GM124165). Use of the APS, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357.
3. CDC. Antibiotic resistance threats in the united states, 2019. U.S. Department of Health and Human Services C (2019). Available at: https://www.cdc.gov/drugresistance/biggest-threats.html.
22. Chen B, Liu Z, Perry K, Jin R. Structure of the glucosyltransferase domain of tcda in complex with rhoa provides insights into substrate recognition. Sci Rep (2022) 12(1):9028. doi: 10.1038/s41598-022-12909-8
32. Kroh HK, Chandrasekaran R, Rosenthal K, Woods R, Jin X, Ohi MD, et al. Use of a neutralizing antibody helps identify structural features critical for binding of clostridium difficile toxin tcda to the host cell surface. J Biol Chem (2017) 292(35):14401–12. doi: 10.1074/jbc.M117.781112
49. Chen P, Jin R. Receptor binding mechanisms of clostridioides difficile toxin b and implications for therapeutics development. FEBS J (2021). doi: 10.1111/febs.16310
51. Kroh HK, Chandrasekaran R, Zhang Z, Rosenthal K, Woods R, Jin X, et al. A neutralizing antibody that blocks delivery of the enzymatic cargo of clostridium difficile toxin tcdb into host cells. J Biol Chem (2018) 293(3):941–52. doi: 10.1074/jbc.M117.813428
59. Lam K-H, Perry K, Shoemaker CB, Jin R. Two vhh antibodies neutralize botulinum neurotoxin E1 by blocking its membrane translocation in host cells. Toxins (Basel) (2020) 12(10):616. doi: 10.3390/toxins12100616




 8613371530291
8613371530291