rongsheng jin lab for sale

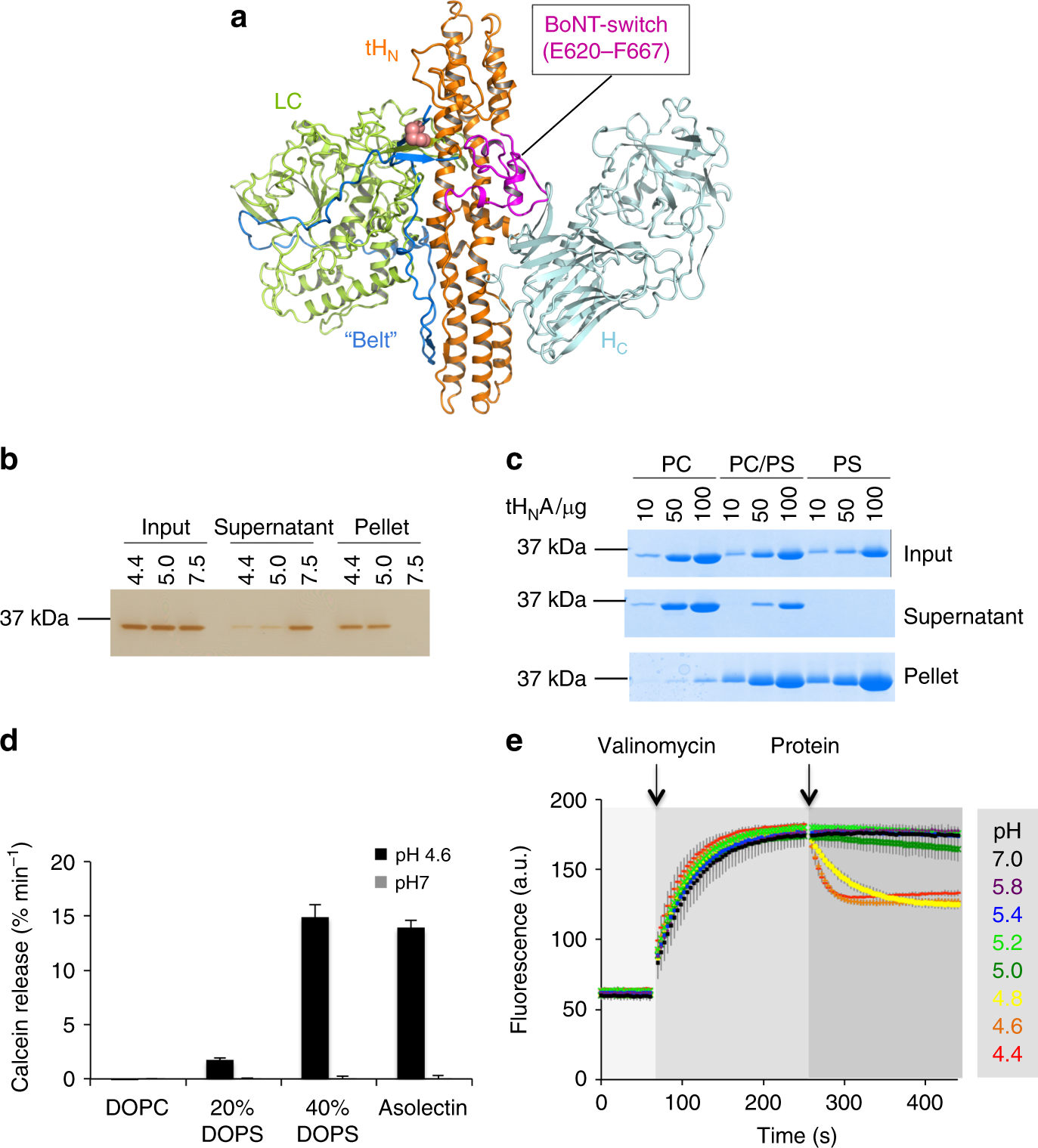
Lam, K. H., Guo, Z., Krez, N., Matsui, T., Perry, K., Weisemann, J., Rummel, A., Bowen, M. E. & Jin, R. A viral-fusion-peptide-like molecular switch drives membrane insertion of botulinum neurotoxin A1. Nat Commun 9, 5367 (2018) doi: 10.1038/s41467-018-07789-4.
Chen, P., Tao, L., Liu, Z., Dong, M. & Jin, R. Structural insight into Wnt signaling inhibition by Clostridium difficile toxin B. FEBS J (2018) doi: 10.1111/febs.14681.
Chen, P., Tao, L., Wang, T., Zhang, J., He, A., Lam, K. H., Liu, Z., He, X., Perry, K., Dong, M*. & Jin, R*. Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B. Science 360, 664-669 (2018) (*corresponding authors) doi: 10.1126/science.aar1999. PMCID: PMC6231499
Lam, K. H., Sikorra, S., Weisemann, J., Maatsch, H., Perry, K., Rummel, A., Binz, T. & Jin, R. Structural and biochemical characterization of the protease domain of the mosaic botulinum neurotoxin type HA. Pathog Dis 76 (2018) doi: 10.1093/femspd/fty044. PMCID: PMC5961070
Silva, D. A., Stewart, L., Lam, K. H., Jin, R. & Baker, D. Structures and disulfide cross-linking of de novo designed therapeutic mini-proteins. FEBS J 285, 1783-1785 (2018) doi: 10.1111/febs.14394. PMCID: PMC6001749
Lam, K. H., Qi, R., Liu, S., Kroh, A., Yao, G., Perry, K., Rummel, A. & Jin, R. The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein. Toxicon 147, 19-26 (2018) doi: 10.1016/j.toxicon.2017.10.012. PMCID: PMC5902665
Chevalier, A., Silva, D.A., Rocklin, G.J., Hicks, D.R., Vergara, R., Murapa, P., Bernard, S.M., Zhang, L., Lam, K.H., Yao, G., Bahl, C.D., Miyashita, S.I., Goreshnik, I., Fuller, J.T., Koday, M.T., Jenkins, C.M., Colvin, T., Carter, L., Bohn, A., Bryan, C.M., Fernández-Velasco, D.A., Stewart, L., Dong, M., Huang, X., Jin, R., Wilson, I.A., Fuller, D.H. & Baker, D. Massively parallel de novo protein design for targeted therapeutics. Nature 550(7674):74-79 (2017) doi: 10.1038/nature23912. PMCID: PMC5802399
Yao, G., Lam, K.H., Weisemann, J., Peng, L., Krez, N., Perry, K., Shoemaker, C.B., Dong, M., Rummel, A. & Jin, R. A camelid single-domain antibody neutralizes botulinum neurotoxin A by blocking host receptor binding. Sci Rep. 7;7(1):7438. (2017) doi: 10.1038/s41598-017-07457-5. PMCID: PMC5547058
Yao, G., Lam, K.H., Perry, K., Weisemann, J., Rummel, A. & Jin, R. Crystal Structure of the Receptor-Binding Domain of Botulinum Neurotoxin Type HA, Also Known as Type FA or H. Toxins (Basel) 9, 93 (2017) doi: 10.3390/toxins9030093. PMCID: PMC5371848
Yao, G., Zhang, S., Mahrhold, S., Lam, K. H., Stern, D., Bagramyan, K., Perry, K., Kalkum, M., Rummel, A.*, Dong, M.* & Jin, R.* N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat Struct Mol Biol 23 (7):656-662 (2016) (*corresponding authors) doi: 10.1038/nsmb.3245. PMCID: PMC5033645
Lee, K., Lam, K. H., Kruel, A. M., Mahrhold, S., Perry, K., Cheng, L. W., Rummel, A. & Jin, R. Inhibiting oral intoxication of botulinum neurotoxin A complex by carbohydrate receptor mimics. Toxicon 107, 43-49 (2015) doi: 10.1016/j.toxicon.2015.08.003. PMCID: PMC4658216
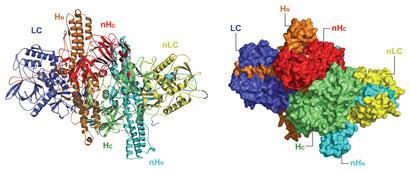
Lam, K.H. & Jin, R. Architecture of the botulinum neurotoxin complex: a molecular machine for protection and delivery. Current Opinion in Structural Biology 31:89-95 (2015) doi: 10.1016/j.sbi.2015.03.013. PMCID: PMC4476938
Lam, K.H., Yao, G. & Jin, R. Diverse binding modes, same goal: The receptor recognition mechanism of botulinum neurotoxin. Progress in Biophysics and Molecular Biology 117(2-3):225-31 (2015) doi: 10.1016/j.pbiomolbio.2015.02.004. PMCID: PMC4417461
Lam, T.I., Stanker, L.H., Lee, K., Jin, R. & Cheng, L.W. Translocation of botulinum neurotoxin serotype A and associated proteins across the intestinal epithelia. Cellular Microbiology 17(8):1133-1143 (2015) doi: 10.1111/cmi.12424. PMCID: PMC4610714
Matsui, T.*, Gu, S., Lam, K.H., Carter, L.G., Rummel, A., Mathews, II. & Jin, R.* Structural Basis of the pH-Dependent Assembly of a Botulinum Neurotoxin Complex. J. Mol. Biol. 426(22):3773-3782 (2014) doi: 10.1016/j.jmb.2014.09.009. (*corresponding authors) PMCID: PMC4252799
Lee, K., Zhong, X., Gu, S., Kruel, A.M., Dorner, M.B., Perry, K., Rummel, A., Dong, M. & Jin, R. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science 344(6190):1405-1410 (2014) doi: 10.1126/science.1253823. PMCID: PMC4164303
Lee, K., Lam, K.H., Kruel, A.M., Perry, K., Rummel, A. and Jin, R. High-resolution crystal structure of HA33 of botulinum neurotoxin type B progenitor toxin complex. Biochem. Biophys. Res. Commun. 446(2):568-573 (2014) doi: 10.1016/j.bbrc.2014.03.008. PMCID: PMC4020412
Yao, Y., Lee, K., Gu, S., Lam, K.H. & Jin, R. Botulinum Neurotoxin A Complex Recognizes Host Carbohydrates through Its Hemagglutinin Component, Toxins (Basel) 6(2):624-635 (2014) doi: 10.3390/toxins6020624. PMCID: PMC3942755
Lee, K., Gu, S., Jin, L., Le, T.T.N., Cheng, L.W., Strotmeier, J., Kruel, A.M., Yao, G., Perry, K., Rummel, A.* & Jin, R.* Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLoS Pathog. 9(10): e1003690 (2013) doi:10.1371/journal.ppat.1003690. (*corresponding authors) PMCID: PMC3795040
Zong, Y. and Jin, R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 70(17):3077-88 (2013) doi: 10.1007/s00018-012-1209-9. PMCID: PMC4627850
Gu, S. and Jin, R. Assembly and function of the botulinum neurotoxin progenitor complex. Curr. Top. Microbiol. Immunol. 364:21-44 (2013) doi: 10.1007/978-3-642-33570-9_2. PMCID: PMC3875173
Gu, S., Rumpel, S., Zhou, J., Strotmeier, J., Bigalke, H., Perry, K., Shoemaker, C.B., Rummel, A. & Jin, R. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science 335(6071):977-81 (2012) doi: 10.1126/science.1214270. PMCID: PMC3545708
Zong, Y., Zhang, B., Gu, S., Lee, K., Zhou, J., Yao, G., Figueiredo, D., Perry, K., Mei, L.* & Jin, R.* Structural basis of neuron-specific regulation of postsynaptic differentiation. Gene & Development 26:247-258 (2012) doi: 10.1101/gad.180885.111. (*corresponding authors) PMCID: PMC3278892
Yao, G., Zong, Y., Gu, S., Zhou, J., Xu, H., Mathews, II. & Jin, R. Crystal structure of the glutamate receptor GluA1 amino-terminal domain. Biochem. J. 438(2):255-63 (2011) doi: 10.1042/BJ20110801. PMCID: PMC3296483
Strotmeier, J., Gu, S., Jutzi, S., Mahrhold, S., Zhou, J., Pich, A., Eichner, T., Bigalke, H., Rummel, A.*, Jin, R.* & Binz, T*. The biological activity of botulinum neurotoxin type C is dependent upon novel types of ganglioside binding sites. Mol. Microbiol. 81(1):143-56 (2011) doi: 10.1111/j.1365-2958.2011.07682.x. Epub 2011 Jun 2. (*corresponding authors)
Strotmeier, J., Lee, K., Völker, A.K., Mahrhold, S., Zong, Y., Zeiser, J., Zhou, J., Pich, A., Bigalke, H., Binz, T., Rummel, A.* & Jin, R.* Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 431(2):207-16 (2010) (*corresponding authors)
Jin, R.*, Singh, S.K., Gu, S., Furukawa, H., Sobolevsky, A.I., Zhou, J., Jin, Y. & Gouaux E.* Crystal structure and association behavior of the GluR2 amino-terminal domain. EMBO J. 28(12):1812-23 (2009) (*corresponding authors) PMCID: PMC2699365
Kumar, J., Schuck. P., Jin, R. & Mayer, M.L. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat. Struct. Mol. Biol. 16(6):631-8 (2009) PMCID: PMC2729365
Jin, R., Rummel, A., Binz, T. & Brunger, A.T. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 444:1092-5 (2006)
Jin, R., Clark, S., Weeks, A.M., Dudman, J.T., Gouaux, E. & Partin, K.M. Mechanism of positive allosteric modulators acting on AMPA receptors. J. Neurosci. 25(39):9027-36 (2005)
Jin, R., Junutula, J.R., Matern, H.T., Ervin, K.E., Scheller, R.H. & Brunger, A.T. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 24:2064-74 (2005)
Jin, R., Bank, T., Mayer, M. L., Traynelis, S. & Gouaux, E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 6(8):803-10 (2003)

Jahid S, Ortega JA, Vuong LM, Acquistapace IM, Hachey SJ, Flesher JL, La Serra MA, Brindani N, La Sala G, Manigrasso J, Arencibia JM, Bertozzi SM, Summa M, Bertorelli R, Armirotti A, Jin R, Liu Z, Chen CF, Edwards R, Hughes CCW, De Vivo M, Ganesan AK. PMID: 35385746; PMCID: PMC9127750.
Chen P, Zeng J, Liu Z, Thaker H, Wang S, Tian S, Zhang J, Tao L, Gutierrez CB, Xing L, Gerhard R, Huang L, Dong M, Jin R. PMID: 34145250; PMCID: PMC8213806.
Chen P, Lam KH, Liu Z, Mindlin FA, Chen B, Gutierrez CB, Huang L, Zhang Y, Hamza T, Feng H, Matsui T, Bowen ME, Perry K, Jin R. PMID: 31308519; PMCID: PMC6684407.

Botulism is caused when the botulinum neurotoxin (BoNT) inhibits the release of a neurotransmitter. The disease can be caused by eating toxin-contaminated food, but how the BoNT protein survives the digestive tract and reaches the bloodstream has been a mystery. Last year, a group led by Rongsheng Jin of the University of California, Irvine, demonstrated how a protein called nontoxic nonhemagglutinin (NTNHA) binds to and shields BoNT to protect it from digestive proteases. Jin and colleagues have now used electron microscopy and X-ray crystallography to study a complex of BoNT, NTNHA, and three hemagglutinin proteins that play a role in getting BoNT past intestinal cells to the blood (PLoS Pathog. 2013, DOI: 10.1371/journal.ppat.1003690). The researchers find that the 760-kilodalton complex evokes the construction of the Apollo lunar lander, with BoNT and NTNHA on top and the hemagglutinins forming “legs,” which are the parts that interact with intestinal epithelial cells. The legs land on and bind to sugars on the cells, facilitating passage of BoNT. Jin and colleagues find that dosing mice with a monosaccharide can reduce BoNT toxicity, suggesting a way to prevent—but not treat—botulism. The full protein complex could also point to ways to deliver protein drugs orally.

A group led by Rongsheng Jin, a neuroscience professor at Sanford-Burnham Medical Research Institute in La Jolla, Calif., has now solved the crystal structure of an inactivated botulinum neurotoxin complexed to its protector NTNHA. The structure shows that NTNHA largely surrounds the part of the toxin involved in binding neuron receptors and moving through membranes. The two proteins associate through electrostatic interactions between a positively charged toxin surface and a negatively charged NTNHA surface, the researchers found.
The work won’t lead directly to a treatment for botulism, because symptoms appear only once the toxin reaches neurons. But a way to disrupt toxin-NTNHA association could stop the disease in the face of a potential outbreak, Jin says. It could also inspire new oral delivery methods for protein pharmaceuticals, such as by combining a therapeutic, a toxin fragment, and NTNHA.
Wild type and mutated recombinant full-length activated BoNT/A1 were produced under biosafety level 2 containment (project number GAA A/Z 40654/3/123) recombinantly in K12 E. coli strain in Dr. Rummel’s lab6-tag were purified on Co2+-Talon matrix (Takara Bio Europe S.A.S., France) and eluted with 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 250 mM imidazole. For proteolytic activation, BoNT/A1 was incubated for 16 h at room temperature with 0.01 U bovine thrombin (Sigma-Aldrich Chemie GmbH, Germany) per µg of BoNT/A1. Subsequent gel filtration (Superdex-200 SEC; GE Healthcare, Germany) was performed in phosphate buffered saline (pH 7.4).
The purified ciA-D12 (S124C) was labeled with Alexa Fluor C2 647 maleimide (Molecular Probes) according to the manufacturer’s instructions. The labeled ciA-D12 was further purified by MonoQ ion-exchange chromatography in 10 mM Hepes (pH 8.0) and eluted with a NaCl gradient. The calculated dye to protein ratio was ~1 mole of dye per mole of ciA-D12. BoNT/A1i-ciA-D12 complex were prepared by mixing BoNT/A1i and ciA-D12 in 1:1.5 molar ratio and the complex was purified by size-exclusion chromatography using Superdex-200.
Liposomes containing 10% GT1b, 69% DOPC, 20% DOPS, 0.5% rhodamine-PE, and 0.5% biotin-PE were prepared by extrusion through a 100 nm pore membrane. To form proteoliposomes, 10 nM BoNT/A1i–D12* or oxidized BoNT/A1iDS–D12* was incubated with 0.5 mg/ml lipid at room temperature for 1 h at the pH indicated. The mixture was then diluted 1000-fold and incubated for 5 minutes in a passivated, quartz microscope chamber functionalized with streptavidin. The biotinylated liposomes were retained and unbound proteins are washed away by exhaustive rinsing with buffer. At the low densities needed for optical resolution of individual liposomes, we could observe sufficient liposomes for statistical analysis, while minimizing the probability that a diffraction-limited spot would contain multiple liposomes. Samples were imaged using a prism-based Total Internal Reflection Fluorescence (TIRF) microscope. Samples were first excited with a laser diode at 640 nm (Newport Corporation, Irvine, CA) to photobleach Alexa 647-labeled BoNT/A1i–D12* or BoNT/A1iDS-D12* molecules followed by excitation with a diode pumped solid state laser at 532 nm (Newport Corporation, Irvine, CA) to probe for Rhodamine-labeled liposomes. Emission from protein and lipids was separated using an Optosplit ratiometric image splitter (Cairn Research Ltd., Faversham UK) containing a 645 nm dichroic mirror, a 585/70 band pass filter for Rhodamine, and a 670/30 band pass filter for Alexa 647 (all Chroma, Bellows Falls, VT). The replicate images were relayed to a single iXon EMCCD camera (Andor Technologies, Belfast, UK) at a frame rate of 10 Hz. Data were processed in MATLAB to cross-correlate the replicate images and extract time traces for diffraction-limited spots with intensity above baseline. Single-molecule traces were hand selected based on the exhibition of single-step decays to baseline during 640 illumination and the appearance of Rhodamine emission during 532 illumination.

Songhai Tian, Xiaozhe Xiong, Ji Zeng, Siyu Wang, Benjamin Jean-Marie Tremblay, Peng Chen, Baohua Chen, Min Liu, Pengsheng Chen, Kuanwei Sheng, Daniel Zeve, Wanshu Qi, David T. Breault, César Rodríguez, Ralf Gerhard, Rongsheng Jin, Andrew C. Doxey, Min Dong
Herbert said that Allergan “almost got started accidentally” and recounted how he’d worked on developing an oral antibiotic with a partner in a lab created in his father’s drugstore balcony on Wilshire Boulevard in Los Angeles.
“Another ophthalmologist helped us with an idea of a product called Blephamide, which became our No. 1 product when we moved to Orange County. In ’61, we had our first real lab production facility, 30,000 square feet in Santa Ana.
UC Irvine associate professor of physiology and biophysics Rongsheng Jin and colleagues found that a series of botulinum neurotoxin compounds bind with patients’ epithelial cell proteins. That initiates a process that disrupts intercellular seals
“By identifying this … process by which the toxin compound manages to open the door from inside, we can better understand how to seek new methods to prevent these deadly toxins from entering the bloodstream,” Jin said in a news release.
Researchers from Harvard University, Hannover Medical School’s Institute for Toxicology in Germany, the Center for Biological Threats and Special Pathogens in Berlin, and the Argonne National Laboratory joined Jin and other UCI faculty in working on the project.

Using a crystal structure of a complex protein compound of botulinum neurotoxin, Rongsheng Jin, associate professor of physiology & biophysics at UC Irvine, and collaborators found that these compounds -- called clostridial hemagglutinin (HA) -- bind with epithelial cell proteins in the intestines of patients, which initiates a process that disrupts the close intercellular seals so that the complex toxin molecules can slip through the epithelial barrier.
"Normally, botulinum neurotoxin molecules are too large to break through this tight junction of epithelial cells," Jin said. "By identifying this novel process by which the toxin compound manages to open the door from inside, we can better understand how to seek new methods to prevent these deadly toxins from entering the bloodstream."
Remarkably, even though this lab-made toxin compound contains the fully active live toxin molecule, it was not orally toxic when tested on mice because the mutated HA cannot break up the intercellular seals and, therefore, the toxin compound cannot be absorbed through the epithelial layer.
Jin said this approach could lead to the identification of small molecules able to stop HA from binding with epithelial cell proteins, thus preventing the toxin invasion.
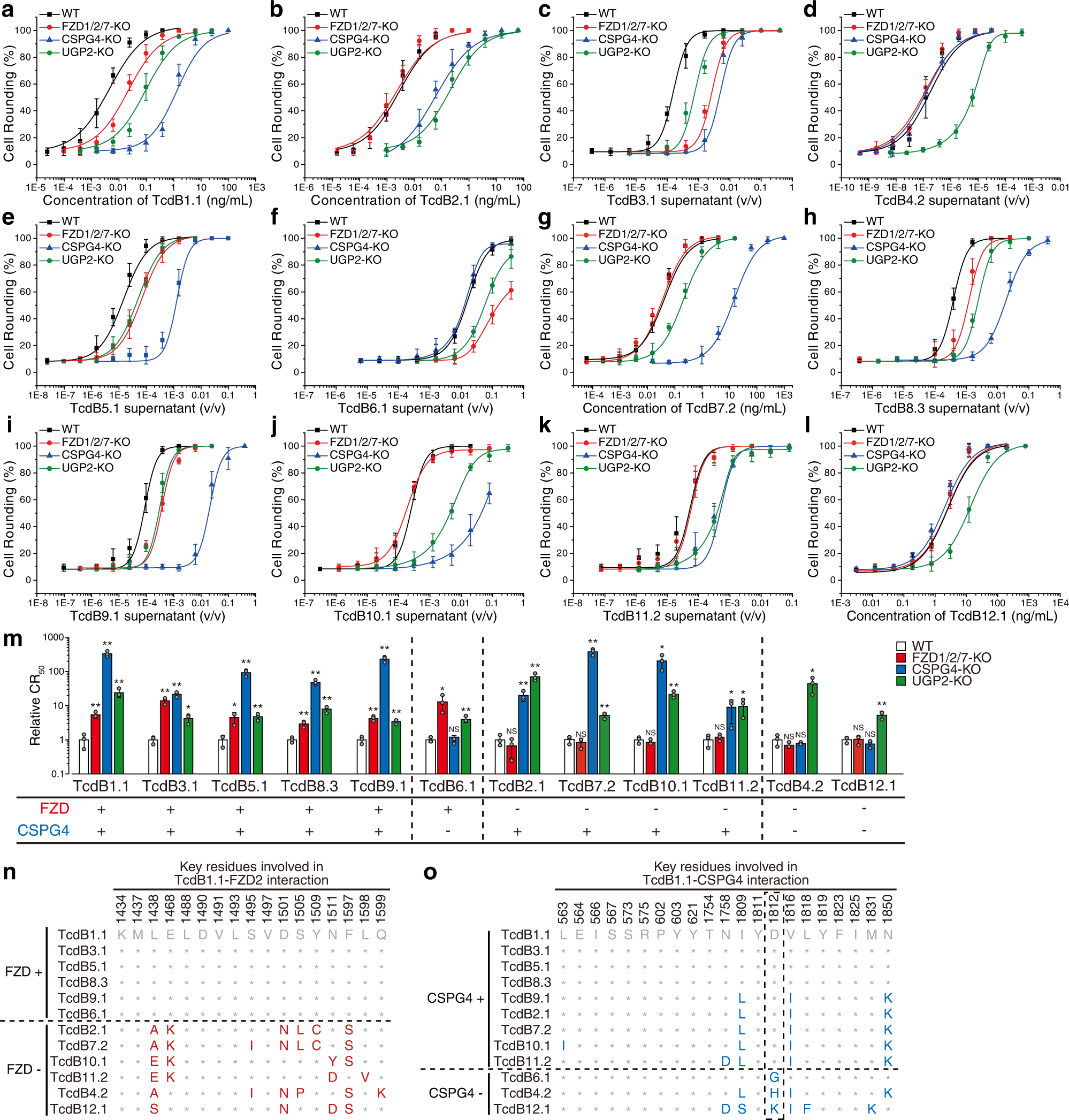
Published today in Nature Communications, the study reveals the first 3D structure of the Clostridioides difficile toxin B (TcdB) in complex with chondroitin sulfate proteoglycan 4 (CSPG4), a human receptor. The study was co-led by senior author Rongsheng Jin, PhD, a professor in the Department of Physiology & Biophysics at the University of California, Irvine, School of Medicine, and Min Dong, PhD, an associate professor at Harvard Medical School.
"TcdB is one of two homologous C. difficile exotoxins, which are major virulence factors responsible for the spread of C. difficile infections," explained Jin. "TcdB alone is capable of causing the full-spectrum of diseases associated with CDI in humans."
"What these new findings tell us is that a rationally designed CSPG4-mimicking decoy could neutralize major TcdB variants, providing a unique therapeutic avenue for combating some of the hypervirulent C. difficile strains," said Jin. In contrast, researchers also revealed that the therapeutic mechanism for bezlotoxumab, the only FDA approved anti-TcdB antibody, is sensitive to escaping mutations in some bacterial strains.
"We have designed a CSPG4-mimicking decoy based on the 3D structure we observed, which could neutralize major TcdB variants and is superior to bezlotoxumab on a major TcdB variant from a hypervirulent strain (TcdB2) in our studies. As a highly conserved cellular receptor of TcdB, a CSPG4 decoy molecule would be difficult for TcdB to escape, since any mutations that disrupt toxin binding to the decoy would also disrupt binding to its native receptors," said Jin.
"We are now examining the therapeutic features of these novel antitoxin molecules, and we believe they could provide broad-spectrum protection and neutralization against most known TcdB variants, thus improving existing antibody therapeutics for CDI," said Jin, whose team has filed a patent on these neutralizing molecules.




 8613371530291
8613371530291