overshot jaw human free sample
Orthodontic abnormalities characterize by teeth misalignment and jaw displacements or clinical malocclusions. These conditions vary depending on the causes, their severity (types), and especially the problems they present to patients and treatment planning.
Malocclusion causes include genetics (hereditary factors), a difference in the size of the jaws, or the size of a tooth that might not correspond with the jaw. Other causes include congenital disabilities (cleft lip and palate) and extra, lost, impacted, or oddly shaped teeth.
However, parents could help prevent some cases. For instance, thumb sucking, tongue thrusting, using a pacifier or a bottle after year three, wrongly attached dentures or orthodontic appliances, jaw fractures, and mouth tumors.
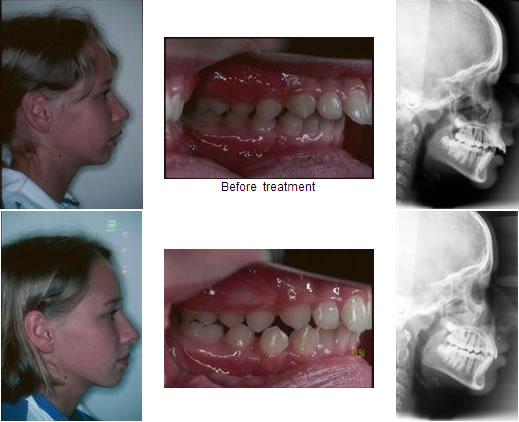
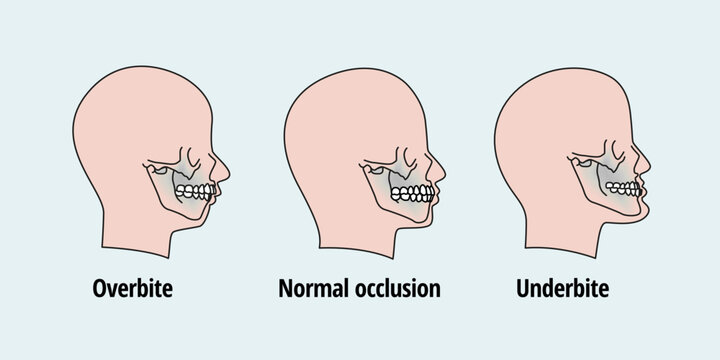
Retrognathism is the clinical term for type two malocclusion. A less technical term frequently used is overbite. The upper jaw significantly overlaps the lower jaw. The mandible doesn’t match the maxilla as it is backward.
The most prominent malocclusion is prognathism. This third type of abnormality, known as an underbite, is characterized by a protruding lower jaw (mandible). This means the lower jaw (mandible) and teeth are mispositioned in front of the upper jaw (maxilla).
An underbite is a dental condition where the lower jaw is mispositioned to the front of the mouth. An underbite can be mild and unnoticeable but still requires orthodontic treatment. However, a severe underbite can be quite evident.
A severe underbite alters the structure of the face making it look disproportionate. The mandible (lower jaw) exceeds the natural boundary set by the maxilla (upper jaw), pushing the lower lips, jaw, and jowl forward.
Not all underbites are the same. In addition, there are different levels of underbites. In a mild case, you might be unable to detect it. In severe cases, the jaw protrudes outward so far that it can be noticeable to others even with the mouth closed.
Genes inheritance is a cause of underbite that escapes a patient’s control. However, in some cases, environmental aspects lead patients, especially children, to develop a protruded jaw, including:
Orthodontic early intervention enhances the chances of avoiding invasive treatment that might include a surgical procedure. Dr. Nima Hajibaik recommends parents bring their kids for an orthodontic consultation at seven while the child’s jaw is still forming, enhancing the possibility of reshaping it.
Braces are the most common mechanism used to correct an underbite. A mild underbite might require the use of braces. However, there might be severe cases where braces are the final step of a more complex treatment involving an Upper Jaw Expander and a Reverse Pull Headgear.
An expander is a device that widens the jaw. The mechanics of an expander includes placing the device in the upper portion of the mouth (roof). Then, with the help of a key, the patient turns the expander to reach a position where both jaws’ widths match ultimately.
The second stage of the treatment requires the patient to use a reverse pull headgear (face mask). First, an orthodontist attaches the expander and the headgear with rubber bands. Then, the mechanism exerts a strain pulling the upper jaw (maxillary) backward.
Severe underbite cases require patients to undergo surgery to re-accommodate the jaw into the desired position. Unfortunately, once the jaw completes its formation process, the number of treatments available to correct underbite decreases, and sometimes surgery is the only option.
The amplitude of the vertical acceleration of the tibia was relatively low in all phases of walking at both speeds (note that the ankle accelerometer shows foot-strikes for only one leg). The high-gain record of the acceleration of the mandible relative to the maxilla shows that during the swing phase of each walking step, there was a small, slow acceleration of the mandible relative to the maxilla: that is, the jaws opened and closed by a small amount as the head moved up and down. This movement was slightly greater at the higher walking speed. However, there were no obvious bursts of masseter EMG with each step, indicating that its stretch reflex was not activated during walking.
During running at both speeds, both the ankle acceleration and the mandibular acceleration following each heel-strike were much larger, as was the vertical displacement of the mandible. The mandible accelerated rapidly downwards about 10 ms after heel-strike (only the alternate heel strikes were registered) and then upwards, and these jaw movements were synchronized with bursts of masseter EMG activity.
During both walking and running, the downward mandibular movement was least on the uphill and greatest during the downhill trials. However, even downhill walking (in which the subject landed on his heels) did not elicit a reflex response in the masseter in this subject. The amplitudes of the reflex responses during running varied with the amplitude of the downward jaw movement induced on the different slopes.
When this subject ran at a moderate pace on a level treadmill, the downward acceleration of the mandible after landing was markedly higher, and the mandible moved about 0.4 mm downwards from its original position. The downward movement then slowed and was followed by a brisk upward movement in which the mandible slightly overshot its original vertical position. This pattern of movement was greater when the subject ran at the same speed on a downwards-sloping treadmill and less when it was inclined upwards. The brisker downward movements during running evoked a burst of activity in the masseter muscles at a latency of about 8 ms (the precise time at which the acceleration began could be estimated only to the nearest millisecond, and the averaging procedure tended to smear the onset time). The amplitude of the EMG response varied with the amplitudes of the mandibular acceleration and position. There was no evidence for inhibitory or longer-latency excitatory reflex responses.
Running at different speeds and on different inclinations of the treadmill led to different patterns of acceleration, velocity and displacement (equivalent to stretch of the jaw-closing muscles) of the head and the mandible. These were clearly the result of the different patterns of gait and forces of landing under these different conditions. For example, subjects running downhill landed on their heels, and therefore landed more forcefully (they also fell a little further). This resulted in increased acceleration of the head and hence relative acceleration of the mandible, compared with running on a level surface. On the other hand, when subjects ran ‘uphill’, they were compelled to land on their toes, and also landed less forcefully (having fallen a smaller distance): this reduced the forces acting on the head and jaw.
The masseter EMG and jaw displacement data from all 12 subjects are summarized in Fig. 4. Walking at the two lowest treadmill speeds produced small amounts of jaw opening and no significant reflex response in the masseter. Running at the two highest treadmill speeds resulted in larger displacements of the mandible, and these were greater for level and downhill slopes than for the uphill slope (Scheffe"s test, P < 0.01). The peak jaw displacement was similar for the two fastest treadmill speeds. Reflex activation of the masseter was seen after landing when running at 2.1 m s−1 and 2.8 m s−1, and this was larger at the higher speed (Scheffe"s test, P < 0.0001). The reflex was smaller when running uphill compared with running downhill (Scheffe"s test, P < 0.05), or on a level surface (Scheffe"s test, P= 0.05).
Data are mean ±s.e.m of 12 subjects. Maximal peak-to-peak (pp) masseter EMG and maximal downward displacement of the mandible after landing were quantified from the averaged records of each subject. Both jaw opening and masseter EMG were larger during running (2.1 and 2.8 m s−1) than walking (0.7 and 1.4 m s−1). Jaw opening and masseteric reflexes were smaller with uphill locomotion than in the level and downhill trials. The masseter reflex responses were larger when running at 2.8 m s−1 compared with 2.1 m s−1, but peak jaw opening did not differ for these running speeds.
The vertical axis of each graph is the averaged peak-to-peak reflex EMG response in the masseter for a given run. The lowermost panel shows that as the running (i.e. treadmill) speed increased beyond a threshold value, the amplitude of the reflex response increased linearly over the running speeds tested. The upper three rows of panels show the relationships between the various parameters of the mandibular movement relative to the mandible (peak acceleration, peak velocity, maximal jaw opening) and the peak-to-peak (pp) amplitude of the reflex responses recorded at the various running locomotor speeds and inclinations. These relationships are clearly non-linear: in many of the graphs, the value of the kinematic parameter initially increases, then begins to diminish as the reflex response increases. This is evidence that the reflex EMG response in the masseter is influencing the kinematics of the jaw movement, restraining its downward movement during locomotion. Data are given for results during •: level locomotion, ○: downhill locomotion and ▾: uphill locomotion.
The purpose of this exploratory study was to determine how jaw movements adapt to whole-head vibration during speech, syllable repetition, and chewing tasks. Although prior work has demonstrated measurable effects of direct vibration on the extent and speed of jaw movements (Hagbarth et al., 1976; Hellsing, 1978; Loucks & De Nil, 2001, 2006; Tsukiboshi et al., 2012), to our knowledge, the effects of whole-head vibration have not been investigated and few studies have examined the vibration response during different jaw motor tasks (Loucks & De Nil, 2001). The findings of this study showed that jaw movements were perturbed by whole- head vibration for some tasks, and the direction and magnitude of the response varied across those tasks. Specifically, the response was observed only during the syllable repetition tasks, and not during speech or chewing. Moreover, the direction of the effect differed for the unconstrained and constrained (target task) syllable repetition tasks; when compared to no vibration condition, the movements of the jaw became larger and faster with vibration during the unconstrained syllable repetition, but slower and smaller with vibration during the target- constrained syllable repetition. These findings suggest that the effect of whole-head vibration on jaw movement may be dependent on task parameters such as spatiotemporal demands, the availability of residual afferent information including auditory, or robust forward models to mitigate the perturbing effects of vibration on proprioception. Additional work is required to understand the implications for assessing jaw sensorimotor integration.
The speed and spatial precision demands, required during the two syllable repetition tasks, may have significantly affected the vibration response. In limb studies, the direction of the response has been found to be task-dependent as task demands may shift control strategies, motor goals, or behavioral targets (Floyd, Holmes, & Dean, 2014; Ivanenko, Grasso, & Lacquaniti, 2000). During the syllable repetition tasks, the participants were instructed to produce the syllables as rapidly as possible. The syllable repetition target task had the extra demand of spatial accuracy as participants were instructed to strike a fixed target during jaw opening for each syllable produced. During the syllable repetition target task, participants were, therefore, required to balance the competing demands of speed and accuracy (e.g., speed - accuracy trade-off, Wickelgren, 1977). We speculate that, during the fixed-target task, the participants prioritized accuracy over speed (Bogacz, Wagenmakers, Forstmann, & Nieuwenhuis, 2010) in the presence of vibration because they tended to undershoot the target as observed by the smaller movements and decreased speed. This undershoot bias is commonly observed in motor learning studies when aiming for a target (Engelbrecht, Berthier, & Sullivan, 2003, Oliveira, Elliott, & Goodman, 2005). This finding of undershoot in response to direct vibration studies is also consistent with other limb studies that have accuracy demands (Capaday & Cooke, 1981; Cordo et al., 1995; Inglis & Frank, 1990) and might be caused by an overestimation of displacement or velocity (Cordo et al., 1995). The perturbing effects of vibration on jaw control during a task that required end-point accuracy also elicited the observed jaw movement undershoot (Loucks & De Nil, 2001, 2006). In contrast, the effects of vibration shifted directions from undershoot to overshoot when the jaw position was free to vary during the unconstrained syllable repetition task. During this task, participants produced larger movements and overshot as compared to the control condition without vibration. We hypothesize that the effort required to achieve the speed demands resulted in this overshoot of jaw end-point position. Limb studies have reported movement overshoots and they have posited that this response is affected by factors such as vibration frequency, location of application of the vibration (i.e., agonist v. antagonistic muscles), timing of the vibration, or demands of the task (Cordo et al., 1995; Eklund, 1972; Kasai, Kawanishi, & Yahagi, 1992; Oliveira at al., 2005).
The absence of a vibration response during chewing also supports the assertion that the neural programs that control simple, overleamed behaviors may be resistant to the addition of proprioceptive noise elicited by the small mechanical loads imposed by whole-head vibration. One hypothesis is that the effect of vibration-related proprioceptive noise is greatest for challenging tasks because they require effortful cognitive control that involves a high level of cortical engagement (Koziol, Budding, & Chidekel, 2011). Unlike the syllable repetition tasks, the speech and chewing tasks were not highly demanding from the perspective that they were familiar, self-paced, and void of externally imposed speed or accuracy demands (i.e., striking a fixed target). Compared to the chewing of food, for example, the chewing of gum does not require motor demands to accommodate changes in bolus consistency and size, as well as the intricate tongue-jaw coordination required to prepare the bolus for swallow. Additional work is needed to determine if a response is elicited during more demanding speech and chewing tasks such as when speaking at a fast rate or when chewing challenging consistencies.
This study presented with several limitations. For the syllable repetition target task, a measure of jaw movement accuracy would have provided additional information about how often participants missed the target in the two experimental conditions (i.e., whole-head vibration and no vibration) and if participants were indeed prioritizing accuracy over speed. For this study, we did not test a range of different parameters (i.e., frequency, amplitude) that may have elicited different vibration responses (Cardinale & Bosco, 2003; Ritzmann, Gollhofer, & Kramer, 2013). The vibration frequency and amplitude used in this study may not have been adequate to perturb the stable jaw movement patterns produced during simple and familiar speaking and chewing tasks. We selected the vibration frequency based on the whole-body vibration literature that recommends 25–45 Hz (Cochrane, 2011b) as well as preliminary pilot testing that showed a robust response at 40 Hz. Finally, although this study has identified several task-dependent effect of vibration on jaw motor function, we acknowledge that the small sample size in this exploratory efficacy study precludes us from making strong conclusions particularly with regard to the non-statistical effects on speech and chewing. The current findings, however, provide the rationale for larger studies and ones that are focused on identifying the vibration parameters that elicit the greatest response.
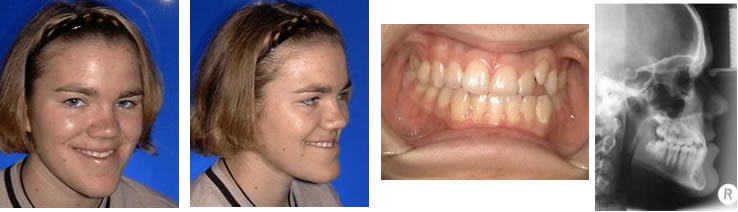
Occlusion refers to the relationship between the maxillary and mandibular teeth when they approach each other, as occurs during chewing or rest. Normal occlusion exists when the maxillary incisors just overlap the mandibular incisors (Figure 1A), the mandibular canines are equidistant from the maxillary third incisors and the maxillary canine teeth, and the premolar crown tips of the lower jaw point between the spaces of the upper jaw teeth in a saw-toothed fashion (Figure 1B). Flat-faced breeds, such as boxers, shih tzus, Boston terriers, Lhasa apsos and Persian cats, have abnormal bites that are recognized as normal for their breed in which the mandibular jaw protrudes in front of the maxillary jaw, altering the above tooth-to-tooth relationship (Figures 2A and 2B).
Malocclusion refers to abnormal tooth alignment. Skeletal malocclusion occurs when jaw anomalies result in abnormal jaw alignment that causes the teeth to be out of normal orientation. Dental malposition occurs when jaw alignment is normal but one or more teeth are out of normal orientation.
Mandibular distoclusion (also called overbite, overjet, overshot, class 2, and mandibular brachygnathism) occurs when the lower jaw is shorter that the upper and there"s a space between the upper and lower incisors when the mouth is closed. The upper premolars will be displaced rostrally (toward the nose) compared with the lower premolars. Mandibular distoclusion is never normal in any breed (Figures 3A and 3B).
Figure 4. Mandibular mesioclusion in a dog.Maxillary mandibular asymmetry (also called wry bite, especially by breeders) is a skeletal malocclusion in which one side of the jaw grows differently from the other side (Figures 5A and 5B).
To achieve strong oral health and a wonderful smile, your upper and lower jaws need to evenly meet. This allows you to do things like eating and swallowing with ease and avoid some very serious health risks to your jaw, mouth and teeth.
Overbites are more common than underbites and are called a Class II bite. One thing to remember is that having a slight overbite is normal because the shape of the human skull naturally allows for the upper teeth to extend beyond the lower teeth. During checkups, your dentist should measure your overbite and underbite and consult with you if they suspect any issues.
An underbite, a Class III bite, is when the lower teeth extend beyond the upper. Essentially, the lower jaw protrudes, making it impossible for the lower row of teeth to align with the upper row causing the potential for several serious oral health issues. This can be caused by the upper jaw bone being underdeveloped or the bone in the lower jaw being overdeveloped.
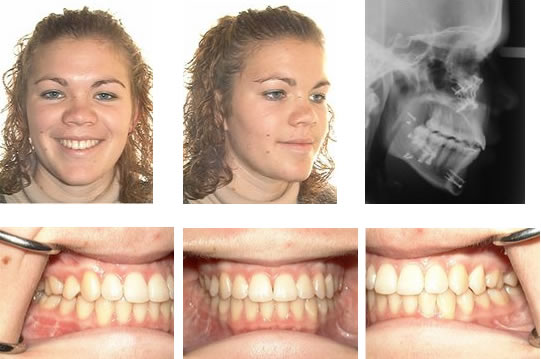
Parrot mouth, long believed to be an inherited condition, reportedly affects 2-5% of the equine population to some degree. Although common in other species, like humans, any degree of overbite is considered abnormal in horses.
“Other animals with an overshot jaw have difficulty grasping food because of misaligned incisors. In horses, incisor malalignment is obvious but not necessarily a significant problem, because they are capable of seizing food with their lips. The major concern is the potential development of cheek-teeth disorders that inhibit their ability to chew,” shared Kathleen Crandell, Ph.D., an equine nutritionist for Kentucky Equine Research.
Foals with an overshot jaw are therefore at risk of malnutrition, slow growth, and the development of additional dental problems. For example, lack of contact between the upper and lower incisors can result in overgrowth of incisors and cheek teeth, known as premolars. In addition, this lack of contact may trap the lower incisors behind the upper incisors, potentially contributing to the lack of lower jaw growth, which exacerbates the condition as the foal grows.
Malocclusions can be a result of abnormal tooth positioning or due to abnormal jaw length relationship. Many animals with malocclusion may have more than one deviation from normal and thus multiple concurrent malocclusions (i.e. mandibular distoclusion with linguoverted mandibular canine teeth). Class 1 malocclusion consists of a normal jaw length relationship with malpositioning of one or more teeth. Generally these malocclusions are described by the physical direction that the tooth is angled or deviated (i.e. mesioverted). Another version of class one malocclusion is a crossbite, where the mandibular teeth are more buccal or labial to the opposing maxillary tooth. Crossbites can be described as rostral crossbite – referring to the incisor teeth, or caudal crossbite – when the malocclusion is associated with the premolars or molars. Other classes of malocclusion deal with skeletal malocclusion.
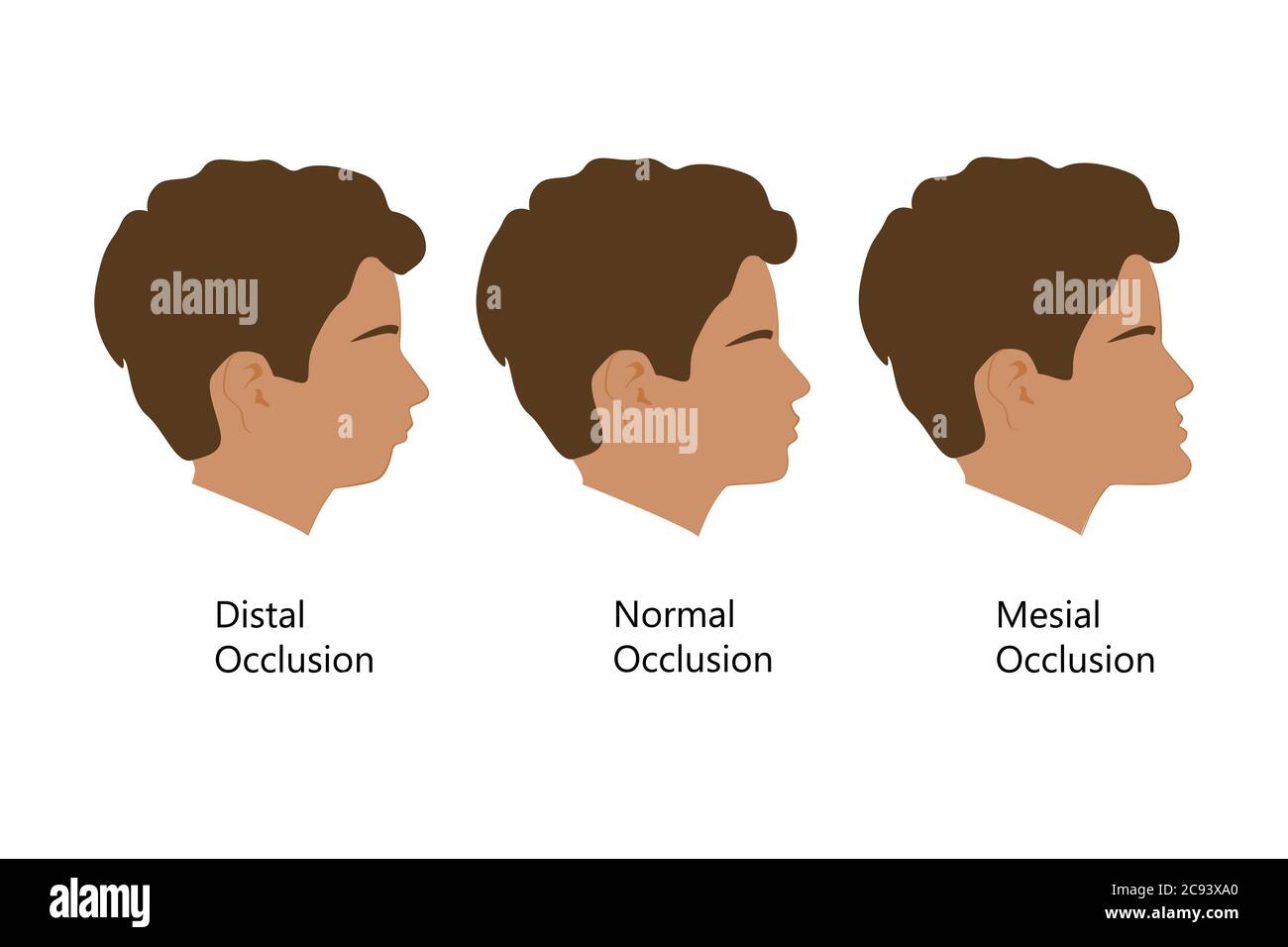
Class 2 malocclusion, or mandibular distoclusion, is when the mandible occludes caudal to the normal position relative to the maxilla. Colloquial terms for this malocclusion include parrot mouth, overbite, overshot jaw. On the other hand, class 3 malocclusion, mandibular mesioclusion, the mandible will be rostral to the maxillary arch. In some breeds this occlusion is normal for the breed (i.e. brachycephalics). Other terms often used are underbite or monkey mouth. Class 4 malocclusions involve asymmetry and can be in a rostro-caudal, side-to-side, dorso-ventral direction, or a combination of these. We try to steer away from the term wry bite, as this is non-specific and does not accurately provide an image of a patient’s malocclusion. An example of class 4 malocclusion in a rostrocaudal direction would be when either the right or left side of the face has mandibular mesioclusion or distoclusion. A side-to-side malocclusion would describe when the midline alignment of the maxilla and mandible is shifted. And finally, a dorsoventral malocclusion would correspond with an open bite in which there is abnormal vertical space between the maxilla and mandible when the mouth is in a closed position.
For many of us, orthodontic work – getting fitted with braces, wearing retainers – was just a late-childhood rite of passage. The same went for the pulling of wisdom teeth in early adulthood. Other common conditions, including jaw pain and obstructed sleep apnea – when slack throat muscles interrupt breathing during rest – also just seem like par for the course.
A new study says that parents and caregivers can take steps to promote proper mouth, jawbone and facial musculature development in children to help stave off future health burdens and chronic conditions.(Image credit: Getty Images)
But in a new study, Stanford researchers and colleagues argue that all these issues and more are actually relatively new problems afflicting modern humans and can be traced to a shrinking of our jaws. Moreover, they maintain that this “jaws epidemic” is not primarily genetic in origin, as previously thought, but rather a lifestyle disease. That means the epidemic is largely the result of human practices and akin to obesity, type 2 diabetes, heart disease and some cancers.
The study – published in the journal BioScience – marshals the growing evidence from studies conducted around the world surrounding the jaws epidemic, as well as how to address it proactively. Parents and caregivers can take steps to promote proper mouth, jawbone and facial musculature development in children, the study advises, to help stave off future health burdens and chronic conditions.
“The jaws epidemic is very serious, but the good news is, we can actually do something about it,” said Paul Ehrlich, the Bing Professor of Population Studies, Emeritus, at Stanford and one of the study’s authors.
The new study builds upon a book Ehrlich co-wrote with orthodontist and lead study author Sandra Kahn entitled Jaws: The Story of a Hidden Epidemic, published by Stanford University Press in 2018. Two other Stanford researchers, Robert Sapolsky and Marcus Feldman, have contributed their expertise to the new study. Seng-Mun “Simon” Wong, a general dentist in private practice in Australia, was also a co-author.
Anthropologists have long noted the significant differences between the jaws and teeth in modern skulls compared to pre-agricultural, hunter-gatherer humans from thousands of years ago. The differences are stark even compared to humans who lived as recently as a century-and-a-half ago during pre-industrial times. These bygone humans showed little teeth crowding, impaction of their wisdom teeth (a leading reason for their surgical removal nowadays) or malocclusion – the abnormal positioning of the upper and lower teeth when the mouth is closed.
Paul Ehrlich wants you to shut your mouth – for your health. According to Ehrlich’s new book, mouth breathing, among other modern habits, has led to an epidemic of small jaws and many troubling health consequences.
Assuming that genetics are chiefly responsible for the sudden modern rise of these dental maladies does not make sense, said Ehrlich. “There’s not been enough time for evolution over the span of only several generations to have made our jaws shrink,” said Ehrlich. Nor is there any evidence of selection pressures that would have favored smaller jawed-people producing more offspring – and thus perpetuating the trait – than regular-jawed people.
“The evidence of a genetic contribution to the jaws epidemic is not strong,” said Feldman, who is a population geneticist and the Burnet C. and Mildred Finley Wohlford Professor and professor of biology.
Instead, profound physiological changes can occur in human populations over short intervals, Feldman pointed out, purely as a result of environmental factors, such as dietary choices and cultural norms. For instance, since World War II, a switchover from heavy rice consumption to more dairy and protein in childhood has been linked to Japanese men gaining around 5 inches in average adult height.
This goes to show that in many cases, lifestyle choices can have just as powerful if not more of an influence on human traits than underlying genetics. “A genetic contribution to a trait, if there is one, does not necessarily sentence you to a life with that trait,” said Feldman. “In almost all cases, you cannot intervene medically to alter a genetic contribution; it’s not actionable. But what is actionable are the things talked about in this study, as well as Paul and Sandra’s book.”
Available evidence points to the jaws epidemic arising as humanity underwent sweeping behavioral changes with the advent of agriculture, sedentism (settling in one place for extended periods) and industrialization. One obvious factor is the softening of diets, especially with the relatively recent invention of processed foods. Also, less chewing is needed nowadays to extract adequate nutrition – our ancestors certainly did not enjoy the sustentative luxury of slurping down protein shakes.
A less obvious, though more significant reason behind the jaws epidemic, Ehrlich and colleagues contend, has been the rise of what they describe as bad oral posture. Our bones grow, develop and change shape under the influences of gentle but persistent pressures, multiple studies have shown. The proper development of the jaw and its associated soft tissues is guided by oral posture – the positioning of the jaws and the tongue during times when children are not eating or speaking. This positioning is especially important overnight during long sleep stretches, when swallowing maintains the correct, gentle pressures. With both children and adults now sleeping on forgiving mattresses and pillows, instead of the firm ground as their ancestors did, mouths are likelier to fall open, disrupting positioning and swallowing.
To promote the proper development of the jaw, the answer is not to start sleeping on rocks. Rather, basic practices such as having children chew sugar-free gum, as well as giving babies less mushy foods as they transition to solid foods, can help, the researchers say. Kahn and Wong also practice what they call forwardontics, which includes exercises such as proper breathing and swallowing patterns to guide jaw growth in children as young as 2 versus waiting until children are older and require more severe interventions. To raise awareness of the jaws epidemic and how to better address it, Ehrlich and his co-authors have been giving lectures to conventions of orthodontists and seen some positive momentum. “There’s no question that some clinical practices are moving in this direction,” said Ehrlich, “but we have a lot more work to do.”
Benefits are not just limited to straighter teeth, roomier jaws and stronger oral muscles. Cutting down on sleep deprivation from sleep apnea is another gain, which has myriad knock-on benefits. Sleep deprivation increases stress, which is associated with greater risks of heart disease, high blood pressure, depression, cancer and Alzheimer’s disease in adult populations, and with attention deficit hyperactivity disorder in children.
“The maladaptive ‘jaws’ profile can disrupt our stress response and ultimately bring about greater stress and chronic activation of the body’s stress response,” said Sapolsky, the John A. and Cynthia Fry Gunn Professor and a professor of biology, of neurology and neurological sciences and of neurosurgery, whose research focuses on stress.
“We’re going to continue learning the causes of the jaws epidemic and continue getting the word out on how this is a highly treatable condition early on in life,” said Ehrlich. “Parents and caregivers, in collaboration with dentists and orthodontists, can all help children to avoid some serious health problems later on in their lives.”

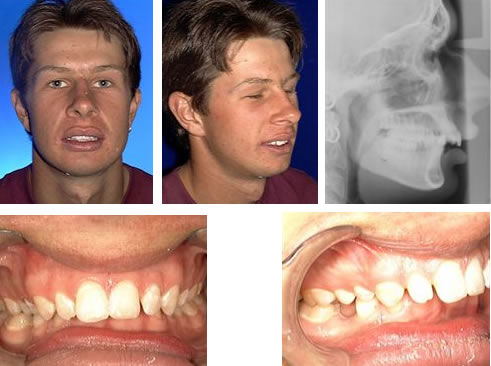
Prognathism, also called Habsburg jaw or Habsburgs" jawHouse of Habsburg,mandible or maxilla to the skeletal base where either of the jaws protrudes beyond a predetermined imaginary line in the coronal plane of the skull.general dentistry, oral and maxillofacial surgery, and orthodontics, this is assessed clinically or radiographically (cephalometrics). The word prognathism derives from Greek πρό (pro, meaning "forward") and γνάθος (gnáthos, "jaw"). One or more types of prognathism can result in the common condition of malocclusion, in which an individual"s top teeth and lower teeth do not align properly.
Mandibular prognathism, where teeth have almost reached their final, straight position by dental braces. This makes the prognathism more obvious, and it will take an operation, moving the jaw backwards, to give the ultimate result.
Prognathism in humans can occur due to normal variation among phenotypes. In human populations where prognathism is not the norm, it may be a malformation, the result of injury, a disease state, a hereditary condition,
Prognathism should not be confused with micrognathism, although combinations of both are found. It affects the middle third of the face, causing it to jut out, thereby increasing the facial area, similar to the phenotype of archaic hominids and other apes. Mandibular prognathism is a protrusion of the mandible, affecting the lower third of the face. Alveolar prognathism is a protrusion of that portion of the maxilla where the teeth are located, in the dental lining of the upper jaw.
Prognathism can also be used to describe ways that the maxillary and mandibular dental arches relate to one another, including malocclusion (where the upper and lower teeth do not align). When there is maxillary or alveolar prognathism which causes an alignment of the maxillary incisors significantly anterior to the lower teeth, the condition is called an overjet. When the reverse is the case, and the lower jaw extends forward beyond the upper, the condition is referred to as retrognathia (reverse overjet).
Pathologic mandibular prognathism is a potentially disfiguring genetic disorder where the lower jaw outgrows the upper, resulting in an extended chin and a crossbite. In both humans and animals, it can be the result of inbreeding.shih tzus and boxers, it can lead to problems such as underbite.
Although more common than appreciated, the best known historical example is Habsburg jaw, or Habsburg or Austrian lip, due to its prevalence in members of the House of Habsburg, which can be traced in their portraits.geneticists and pedigree analysis; most instances are considered polygenic,
Allegedly introduced into the family by a member of the Piast dynasty, it is clearly visible on family tomb sculptures in St. John"s Cathedral, Warsaw. A high propensity for politically motivated intermarriage among Habsburgs meant the dynasty was virtually unparalleled in the degree of its inbreeding. Charles II of Spain, who lived 1661 to 1700, is said to have had the most pronounced case of the Habsburg jaw on record,consanguineous marriages in the dynasty preceding his birth.
Peacock, Zachary S.; Klein, Katherine P.; Mulliken, John B.; Kaban, Leonard B. (September 2014). "The Habsburg Jaw-re-examined". American Journal of Medical Genetics. Part A. 164A (9): 2263–2269. doi:10.1002/ajmg.a.36639. PMID 24942320. S2CID 35651759.
Zamudio Martínez, Gabriela; Zamudio Martínez, Adriana (2020). "A Royal Family Heritage: The Habsburg Jaw". Facial Plastic Surgery & Aesthetic Medicine. 22 (2): 120–121. doi:10.1089/fpsam.2019.29017.mar. PMID 32083497. S2CID 211232475.
Безуглый, Т. А. (2020). "Влияние На Человека Признаков, Передаваемых По Аутосомно-Рецессивному Типу (на Примере Династии Габсбургов)" [Influence on the Human Traits Transmitted According to the Autosomal-Recessive Type (on the Example of the Habsburg Dynasty)] (in Russian).
Vilas, Román; Ceballos, Francisco C.; Al-Soufi, Laila; González-García, Raúl; Moreno, Carlos; Moreno, Manuel; Villanueva, Laura; Ruiz, Luis; Mateos, Jesús; González, David; Ruiz, Jennifer; Cinza, Aitor; Monje, Florencio; Álvarez, Gonzalo (17 November 2019). "Is the "Habsburg jaw" related to inbreeding?". Annals of Human Biology. 46 (7–8): 553–561. doi:10.1080/03014460.2019.1687752. PMID 31786955. S2CID 208536371.




 8613371530291
8613371530291