overshot titration free sample

Sketch out a plot representing the titration of a strong monoprotic acid by a strong base, or of a strong base titrated by a strong acid. Identify the equivalence point and explain its significance.
Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Identify the equivalence point and half-equivalence points.
The objective of an acid-base titration is to determine \(C_a\), the nominal concentration of acid in the solution. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction
A plot showing the pH of the solution as a function of the quantity of base added is known as a titration curve. These plots can be constructed by plotting the pH as a function of either the volume of base added, or the equivalent fraction \(ƒ\) which is simply the number of moles of base added per mole of acid present in the solution. In most of the titration curves illustrated in this section, we plot pH as a function of \(ƒ\). It"s worth taking some time to thoroughly familiarize yourself with the general form of a titration curve such as the one shown below, in which a weak acid HA is titrated with a strong base, typically sodium hydroxide.
A similar effect is seen at the low-pH side of the curve when a strong acid is titrated, as in the plot for the titration of HCl below. In this case, the buffering is due to {H3O+) ≈ {H2O}.
It is important to understand that the equivalent fraction ƒ of base that must be added to reach the equivalence point is independent of the strength of the acid and of its concentration in the solution. The whole utility of titration as a means of quantitative analysis rests on this independence; we are in all cases measuring only the total number of moles of “acidic” hydrogens in the sample undergoing titration.
Although the strength of an acid has no effect on the location of the equivalence point, it does affect the shape of the titration curve and can be estimated on a plot of the curve.
it will be apparent that this equation reduces to pH = pKa when the titration is half complete (that is, when [HA] = [A–]), the pH of the solution will be identical to the pKa of the acid. This equation does not work for strong acids owing to the strong buffering that occurs at the very low pH at which ƒ = 0.5.
It is important to understand the reasons for these two relations. The second is the simplest to explain. Titration of an acid HA with a base such as NaOH results in a solution of NaA; that is, a solution of the conjugate base A–. Being a base, it will react with water to yield an excess of hydroxide ions, leaving a slightly alkaline solution. Titration of a weak base with an acid will have the opposite effect.
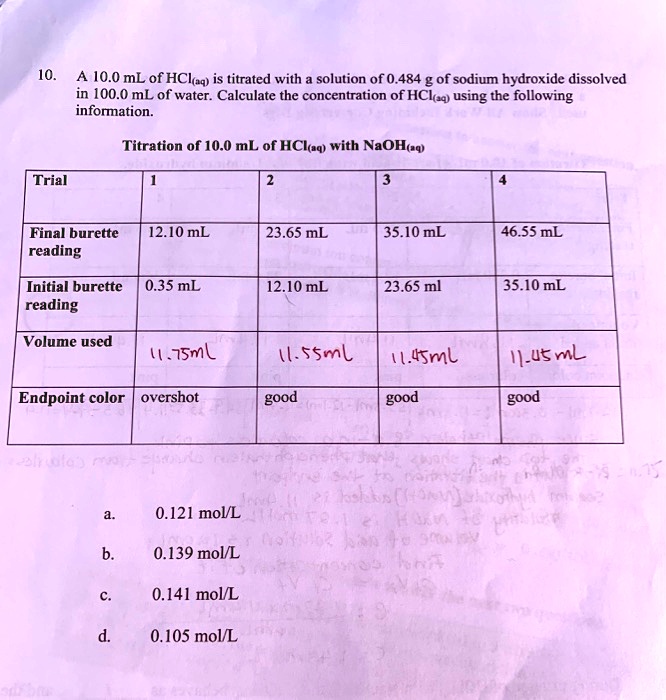
The extent of the jump in the pH at the equivalence point is determined by a combination of factors. In the case of a weak acid, for example, the initial pH is likely to be higher, so the titration curve starts higher. Further, the weaker the acid, the stronger will be its conjugate base, so the higher will be the pH at the equivalence point. These two factors raise the bottom part of the titration curve. The upper extent of the curve is of course limited by the concentration and strength of the titrant.
These principles are clearly evident in the above plots for the titrations of acids and bases having various strengths. Notice the blue curves that represent the titration of pure water (a very weak acid) with strong acid or base.
"Weak/weak" titrations tend to be problematic as the buffered regions move closer to ƒ=1. The equivalence point pH of 7 in these examples reflects the near-equality of pKa and pKb of the reactants.
It can be difficult to reliably detect the equivalence point in the titration of boric acid (pKa = 9.3) or of other similarly weak acids from the shape of the titration curve*. *An interesting student laboratory experiment that employs an auxiliary reagent (mannitol) to make boric acid stronger and thus more readily titratable was described in J. Chem Ed. 2012, 89, 767-770.
The problem here is that aqueous solutions are buffered against pH change at very low and very high pH ranges. An extreme example occurs in the titration of pure water with a strong acid or base. At these extremes of pH the concentrations of H3O+ and of OH– are sufficiently great that a competing buffer system (either H3O+/H2O or H2O/OH–, depending on whether the solution is highly acidic or highly alkaline) comes into play.
In practice, many of the titrations carried out in research, industry, and clinical practice involve mixtures of more than one acid. Examples include natural waters, physiological fluids, fruit juices, wine making, brewing, and industrial effluents. For titrating these kinds of samples, the use of anything other than a strong titrant presents the possibility that the titrant may be weaker than one or more of the "stronger" components in the sample, in which case it would be incapable of titrating these components to completion.
The effect of the first point is seen by comparing the titration curves of two diprotic acids, sulfurous and succinic. The appearance of only one equivalence point in the latter is a consequence of the closeness of the first and second acid dissociation constants. The pKa"s of sulfurous acid (below, left) are sufficiently far apart that its titration curve can be regarded as the superposition of those for two independent monoprotic acids having the corresponding Ka"s. This reflects the fact that the two acidic –OH groups are connected to the same central atom, so that the local negative charge that remains when HSO3– is formed acts to suppress the second dissociation step.
Two other examples of polyprotic acids whose titration curves do not reveal all of the equivalence points are sulfuric and phosphoric acids. Owing to the leveling effect, the apparent Ka1 of H2SO4 is so close to Ka2 = 0.01 that the effect is the same as in succinic acid, so only the second equivalence point is detected.
Whether or not the equivalence point is revealed by a distinct "break" in the titration curve, it will correspond to a unique hydrogen ion concentration which can be calculated in advance. There are many ways of determining the equivalence point of an acid-base titration.
The traditional method of detecting the equivalence point has been to employ an indicator dye, which is a second acid-base system in which the protonated and deprotonated forms differ in color, and whose pKa is close to the pH expected at the equivalence point. If the acid being titrated is not a strong one, it is important to keep the indicator concentration as low as possible in order to prevent its own consumption of OH– from distorting the titration curve.
The observed color change of an indicator does not take place sharply, but occurs over a range of about 1.5 to 2 pH units. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. Some plants contain coloring agents that can act as natural pH indicators. These include cabbage (shown), beets, and hydrangea flowers.
For a strong acid - strong base titration, almost any indicator can be used, although phenolphthalein is most commonly employed. For titrations involving weak acids or bases, as in the acid titration of sodium carbonate solution shown here, the indicator should have a pK close to that of the substance being titrated.
A more modern way of finding an equivalence point is to follow the titration by means of a pH meter. Because it involves measuring the electrical potential difference between two electrodes, this method is known as potentiometry. Until around 1980, pH meters were too expensive for regular use in student laboratories, but this has changed; potentiometry is now the standard tool for determining equivalence points.
Plotting the pH after each volume increment of titrant has been added can yield a titration curve as detailed as desired, but there are better ways of locating the equivalence point. The most common of these is to take the first or second derivatives of the plot: d(pH)/dV or d2(pH)/dV2 (of course, for finite increments of pH and volume, these terms would be expressed as Δ(pH)/ΔV and Δ2(pH)/ΔV2 .)
The idealized plots shown above are unlikely to be seen in practice. When the titration is carried out manually, the titrant is added in increments, so even the simple titration curve
Monitoring the pH by means of an indicator or by potentiometry as described above are the standard ways of detecting the equivalence point of a titration. However, we have already seen that in certain cases involving polyprotic acids or bases, some of the equivalence points are obscured by their close proximity to others, or by the buffering that occurs near the extremes of the pH range. Similar problems can arise when the solution to be titrated contains several different acids, as often happens when fluids connected with industrial processes must be monitored.
See this Wikipedia page for more on thermometric titrations, including many examples. Note also the video on this topic in the "Videos" section near the end of this page.
Thermometric titrations are not limited to acid-base determinations; they can also be used to follow precipitation-, complex formation-, and oxidation-reduction reactions.
A typical thermometric titration curve consists of two branches, beginning with a steep rise in temperature as the titrant being added reacts with the analyte, liberating heat. Once the equivalence point is reached, the rise quickly diminishes as heat production stops. Then, as the mixture begins to cool, the plot assumes a negative slope.
Acids and bases are electrolytes, meaning that their solutions conduct electric current. The conductivity of such solutions depends on the concentrations of the ions, and to a lesser extent, on the nature of the particular ions. Any chemical reaction in which there is a change in the total quantity of ions in the solution can usually be followed by monitoring the conductance. Acid-base titrations fall into this category. Consider, for example, the titration of hydrochloric acid with sodium hydroxide. This can be described by the equation
which shows that two of the four species of ions being combined disappear at the equivalence point. During the course of the titration, the conductance of the solution falls as H+ and Cl– ions are consumed. At the equivalence point the conductance passes through a minimum, and then rises as continued addition of titrant adds more Na+ and OH– ions to the solution.
However, because the conductances of individual ions cannot be observed directly, conductance measurements always register the total conductances of all ions in the solution. The change in conductance that is actually observed during the titration of HCl by sodium hydroxide is the sum of the ionic conductances shown above.
For most ordinary acid-base titrations, conductimetry rarely offers any special advantage over regular volumetric analysis using indicators or potentiometry. This is especially true if the acid being titrated is weak; if the pKa is much below 2, the rising salt line (Na+ when titrating with NaOH) will overwhelm the fall in the contribution the small amount of H+ makes to the conductance, thus preventing any minimum in the total conductance curve from being seen.
These examples illustrate two unique capabilities of conductimetric titrations: (left) Titration of a mixture of two acids and (right) Titration of a strong polyprotic acid →
In four years of college lab sessions, many Chemistry majors will likely carry out fewer than a dozen titrations. However, in the real world, time is money, and long gone are the days when technicians were employed full time just to titrate multiple samples in such enterprises as breweries, food processing (such as blending of canned orange juice), clinical labs, and biochemical research.
A titration is carried out by adding a sufficient volume \Vo of the titrant solution to a known volume \Vt of the solution being titrated. This addition continues until the end point is reached. The end point is our experimental approximation of the equivalence point at which the acid-base reaction is stoichiometrically complete (ƒ = 1). The quantity we actually measure at the end point is the volume V_ep of titrant delivered to the solution undergoing titration.
Equation \ref{3-3} is important!In any titration, both the volume and the concentration of the titrant are known, so the unknown concentration is easily calculated.
In titrations carried out in the laboratory, the titrant is delivered by a burette that is usually calibrated in milliliters, so it is more convenient to express MHA in millimoles and CHA in millimoles/mL (mMol ml–1); note that the latter is numerically the same as moles/L.
This page titled 13.5: Acid/Base Titration is shared under a CC BY 3.0 license and was authored, remixed, and/or curated by Stephen Lower via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.

Not sure what titration is or what you can do with it? Then you are in the right place! In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed and some tips to get better results.
Titration is a method commonly used in chemistry to figure out the amount of a chemical in a solution. This chemical is called the titrand. To figure out the amount of titrand in the solution, a known amount of a different chemical is added to the titrand"s solution; this chemical— called the titrant, or titrating solution—reacts with the titrand. By measuring how much of the titrating solution is needed to react with all of the titrand in the solution, you can calculate how much titrand was in the solution. Simply put:
You can watch the video below, made by the Massachusetts Institute of Technology (MIT)"s Department of Chemistry, to see titration in action. Note: This video uses an indicator that turns light pink at the endpoint, but different indicators turn different colors at their endpoints. The next section contains more information about indicators.
There are many different types of indicators used in titration experiments. Which indicator is used depends on the chemistry of the reaction taking place between the titrand and the titrating solution. This titration tutorial will cover two commonly used indicators—starch and phenolphthalein—along with their associated reactions.
Starch is an indicator that reacts with iodine. When there is iodine present, starch reacts with it to create a blue chemical complex. This means the solution turns blue! How is this used in titration? Iodine is included in the titrating solution, and as it is added to the titrand"s solution (which includes the titrand and starch), the titrand reacts with the iodine to turn it into iodide ions (which do not react with the starch). However, as soon as all of the titrand has reacted with the iodine and the endpoint is reached, the addition of any more iodine will finally react with the starch and turn the titrand"s solution blue!
An example of titration using a starch indicator is the titration of vitamin C, which is technically ascorbic acid. Ascorbic acid reacts with iodine to make dehydroascorbic acid and iodide ions. (This reaction is technically an oxidation-reduction reaction, also called a redox reaction for short.) When ascorbic acid and starch are both in a solution, iodine will react with the ascorbic acid. So when titrating ascorbic acid, a titrating solution containing iodine is added to the titrand"s solution, which contains starch (the indicator) and ascorbic acid (the titrand), and when all of the ascorbic acid has reacted with the iodine, any more iodine added will react with the starch and turn the titrand"s solution blue! Figure 1, below, shows a picture of the endpoint of an ascorbic acid titration using starch and iodine. Because there is a known concentration of iodine in the titrating solution, by keeping track of how much solution is added, you can determine how much titrand there was.
Figure 1. The titrand"s solution turns blue-black when the endpoint has been reached in a titration using starch as an indicator (to react with iodine).
Acids, Bases, & the pH Scale.) Specifically, phenolphthalein is colorless when the pH of a solution is acidic or neutral, but when the solution becomes slightly basic, phenolphthalein turns slightly pinkish, and then darker pink as the solution becomes more basic. How is this used in titration? A base is included in the titrating solution, and it is added to the titrand"s solution, which contains an acidic titrand and phenolphthalein. As more base is added to the titrand"s solution, the pH changes, becoming more basic, and the solution changes color. Usually, with this indicator, when the titrand"s solution just starts to turn pink, you have reached the endpoint.
An example of titration usng phenolphthalein is the titration of vinegar, which is technically acetic acid. When titrating acetic acid, a titrating solution containing a base—normally sodium hydroxide—is added to the titrand"s solution, which contains phenolphthalein (the indicator) and acetic acid (the acidic titrand). (The acetic acid reacts with the sodium hydroxide in an acid-base reaction.) When the titrand"s solution becomes basic enough due to the addition of the basic titrating solution, the phenolphthalein turns the titrand"s solution slightly pink. Phenolphthalein is specifically colorless at a neutral or acidic pH, and becomes light pink as the pH becomes more basic (first turning slightly pink around a pH of 8.3). Figure 2, below, shows a picture of the endpoint of an acetic acid titration using phenolphthalein and sodium hydroxide. Because the number of moles of sodium hydroxide used to titrate the acetic acid equals the number of moles of acetic acid in the titrand solution, by keeping track of how much titrating solution is added, you can determine how much titrand there was.
Figure 2. The titrand"s solution turns slightly pink when the endpoint has been reached in a titration using phenolphthalein as an indicator (to show the change in pH).
There are many steps that should be taken to ensure that a titration is successful and that the results produced are accurate. Check out the video of best practices in titration. Here are some key points to follow and keep in mind when doing a titration:
Assembling the titration setup. Figure 3, below, shows what the general titration setup should look like. The buret is held in place by the buret clamp, which is attached to the ring stand. The titrand"s solution should be placed directly under the bottom of the buret, as shown in Figure 4, below. The buret, which can be moved up and down, should be adjusted so that it is just above the opening of the flask containing the titrand"s solution, as shown in Figure 4.
Figure 3. This picture shows a general titration setup. Note that the buret clamp is firmly attached to the ring stand. The buret shown here slides into place between the prongs of the buret clamp. The buret is held firmly in place, but can be moved up and down if needed.
Adding the titrating solution to the titrand"s solution. Using the red stopper at the bottom of the buret, slowly add the titrating solution to the titrand"s solution one drop at a time. It is important to only let the titrating solution be added one drop at a time because the titration reaction can be very sensitive. One drop may be enough to drive the reaction to completion (if it was near completion before). If more than one drop is added at a time, the data may not be as accurate as it could be. After each drop is added, swirl the flask to mix in the titrating solution. When adding the titrating solution, you may see a temporary color change that goes away when you swirl the flask, as shown in Figure 8, below. If this happens, continue adding one drop at a time; you have reached the titration endpoint when there is a more lasting color change throughout the entire titrand"s solution, as shown in
Watching for the endpoint. Add the titrating solution, mixing in one drop at a time by swirling the flask, until a color is seen throughout the solution that lasts for longer than 20 seconds. At this point, you have reached the endpoint and the titration is complete. If you are using starch as an indicator, your endpoint may look similar to Figure 1, whereas if you are using phenolphthalein as an indicator, your endpoint may look similar to Figure 2. Note that different indicators will cause the endpoint to have a different color; you should confirm the expected color of your endpoint before starting the titration. Lastly, it is important to not overshoot the endpoint (by adding too much titrating solution) because this can cause your results to be inaccurate.
Troubleshooting: No color change is seen. There are a number of reasons why a titration may not work. Here are the most common problems that can lead to a titrand"s solution not changing colors:
Using incorrect concentrations. If you are performing an ascorbic acid titration and the standard solution is too concentrated, or your titrating solution is too diluted, or your indicator solution is not the correct concentration, it may require more than 50 mL of iodine solution to titrate the sample. Note: When doing an ascorbic acid titration, the most common problem here is an overly diluted iodine solution; sometimes the Lugol"s iodine solution sold in stores is already diluted and you do not need to dilute it more.
In the example titration using phenolphthalein in the titration of acetic acid, the unknown amount of acetic acid (the titrand) can again be determined by setting up a proportion with the known amount of sodium hydroxide (the titrating solution). Specifically, the number of moles of sodium hydroxide used to titrate the acetic acid equals the number of moles of acetic acid in the titrand"s solution. For example, if you added 12.5 mL (0.0125 liters [L]) of a 0.1 molar (M, which is moles/L) sodium hydroxide to titrate the acetic acid, the number of moles of both sodium hydroxide and acetic acid would be 0.0125 L x 0.1 moles/L = 0.00125 moles. You could divide by the amount of the sample (in liters) to determine the molar concentration of the acetic acid. For example, if your sample volume was 1.5 mL (0.0015 L), it would have a molarity of 0.00125 moles / 0.0015 L = 0.833 M.
Tabacco, S. and Siddiqui, A. (2003). The Digital Lab Techniques Manual: Titration. Massachusetts Institute of Technology (MIT). Department of Chemistry. Retrieved November 8, 2013.

5) In this simulated titration, the KHP solution was prepared for you and the precise concentration was given. Which of the following hypothetical errors would have a greater influence on the average NaOH concentration? Making a 1% mistake in preparing the original KHP solution, or overshooting the end point by 1% in one of your titrations?

3. In part B if the endpoint of the titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar?

In chemistry, a primary standard is a reagent that is very pure, stable, not hygroscopic, and has a high molecular weight. Ideally, it’s also non-toxic, inexpensive, and readily available. A primary standard provides a reference to find unknown concentrations in titrations and is used to prepare secondary standards and working solutions.
Chemicals react according to mole ratios. A titration determines the concentration of an unknown solution based on volume of a solution with known concentration needed to react with the solution of unknown concentration. But, the accuracy of the calculation relies on truly knowing the concentration of one solution.
Using a primary standard offers a high degree of confidence in the concentration of the unknown solution. Because this solution has been standardized against the primary standard, it can be used as a secondary standard. The degree of confidence in the concentration is slightly lower because of error in the process (for example, overshooting the mark for a titration). But, for some chemicals, this type of standardization is the best way to get reliable concentration value.
indicator colour change is the end point of the titration. Titration » Titration - end point. What is the equivalence point? thiosulfate (S 2 O 3 2 − ), and when all iodine is spent the blue colour disappears. end point: the point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. A correct endpoint is shown on the left, an overshot endpoint on the right. The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds). Q14. The endpoint of a titration is the point where the indicator just changes colour. Back titrations are also useful if the reaction between the analyte and the titrant is very slow, or when the analyte is in a non-soluble solid. Titration is a procedure of careful addition of one solution to another solution a little at a time until a specificend point is reached. Whereas the equivalence point is a point at which exactly enough amount of titrant neutralizes the analyte. Titration is an analytical laboratory method of determining the molar concentration of an analyte (the solution being identified). What is the equivalence point? In it, titrand is the remaining amount of reagent added in excess. Figure 5 Delivering a stream of titrant. This article explains these concepts along with the key differences between endpoint and equivalence point. When you get to the point where the colour doesn"t change back you have reached the end-point. What is the endpoint and equivalence point of a titration? The end point of a titration is the point at which the indicator changes color. The endpoint of a titration is when the indicator first changes in appearance, or when an instrument first gives a reading which indicates that the titration is finished. The manufacture of soap requires a number of chemistry techniques. After the reaction between the substance and the standard solution is complete, the indicator should give a clear colour change. Click to see full answer. I know that: 0.025 L x 0.1M = 2.5 x 10^-3 moles of NaOH and HCl each. The endpoint of a titration is the point where the indicator just changes colour. endpoint. An endpoint is any device that is physically an end point on a network. What is End Point in Titration. What is end point and equivalence point? During the starting of titration an acid base indicator ( eg: phenophthalein,methylo …. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. Who are the experts? Examples of endpoints include: Desktops. Next lesson. The endpoint is always . titration end point. A) at pH7. In no other way does it differ from the forms of skin testing that have been widely used for generations. …. It comes with or after the equivalence point and is considered an ideal point of end the titration. It is possible to overshoot the endpoint by adding too much titrant. Laptops. Indicator: It is a chemical reagent used to recognize the attainment of end point in a titration. For a strong acid and a strong base such as NaOH and HCl the final solution is neutral at pH 7: HCl_((aq))+NaOH_((aq))rarrNaCl_((aq))+H_2O_((l)) Most indicators . Expert Answer. The endpoint is simply the end of the titration reaction indicated by the change in color of the selected indicator. In this way, how do you find the endpoint of a titration? During titration, a known concentration of a reactant is prepared and gradually added to the analyte, while carefully measuring the volume, until a reaction threshold is reached. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. As we approach the endpoint, we start adding titrants in very small . Key Points. In the case of Ksp, overshooting the titration point with an acid means you are adding more cations than you intended (a higher concentration of . Experts are tested by Chegg as specialists in their subject area. What is end point and equivalence point? It is possible to overshoot the endpoint by adding too much titrant. What is a Titration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is an analytical laboratory method of determining the molar concentration of an analyte (the solution being identified). Sort by: Top Voted. Iodometry, also known as iodometric titration, is a volumetric chemical analysis method based on a redox titration in which the presence or disappearance of elementary iodine is used to determine the endpoint of the titration. The end point is used as an approximation of the equivalence point and is employed, with the known concentration of the titrant, to calculate the amount or concentration of the analyte. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. What happens when you overshoot the end point? What is End Point in Titration. A correct endpoint is shown on the left, an overshot endpoint on the right. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference electrode as a function of titrant volume. The color changes is not definite that"s why sodium diphenylamine . standard solution is equal to the moles of a solution . The end point demonstrates the equivalence point, typically by some form of indicator. 4. The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. The endpoint is when the end of the titration is detected like when phenolphthalein has changed pink. When phenolphthalein is the indicator, the end point will be signified by a faint pink color. It is the point where the analyte has completely reacted with the titrant. Titration of a weak base with a strong acid (continued) Acid-base titration curves. Smartphones. The usefulness of IDT has been called into question by some authors, while others believe that studies demonstrating that SPT was superior might have been subject to bias. Figure 4 Preparing the solution for titration. More often than not, the color change occurs after the equivalence point has already been reached. Back titration is typically applied in acid-base titrations: When the acid or (more commonly) base is an insoluble salt (e.g., calcium carbonate) When direct titration endpoint would be hard to discern (e.g., weak acid and weak base titration) When the reaction occurs very slowly Q14. Click to see full answer Besides, what is the endpoint of a titration? Endpoint titration mode (EP): The endpoint mode represents the classical titration procedure: the titrant is added until the end of the reaction is observed, e.g., by a colour change of an indicator. During titration, a known concentration of a reactant is prepared and gradually added to the analyte, while carefully measuring the volume, until a reaction threshold is reached. Knowing the volume of titrant added allows the determination of the concentration of the unknown. All methods of the end point detection are based on the visible changes of the solution properties. E) when the indicator is yellow The closer the end point to the equivalence point the better, but it is often not easy to find a good method of equivalence point detection. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. The end point typically comes straight after the equivalence point, which is when the moles of a standard solution (titrant) equal the moles of a solution of unknown concentration (analyte), i.e., the ideal point for the completion of titration. Endpoint titration mode (EP): The endpoint mode represents the classical titration procedure: the titrant is added until the end of the reaction is observed, e.g., by a colour change of an indicator. Examples of endpoints include: Desktops. At this point, you have reached the endpoint and the titration is complete. What is End Point in Titration The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. During the process, two important stages known as endpoint and equivalence point are reached. D) Always the same at the equivalence point. Color changes are not instant. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. With an automatic titrator, the sample is titrated until a predefined value is reached, e.g. However, very often we can easily spot a point very close to the equivalence point - and that"s where the end point will be. One necessary piece of information is the saponification number. Smartphones. This is the currently selected item. Scout Titration How is soap made? The endpoint is always . Because volume measurements play a key role in titration, it is also known as volumetric analysis.A reagent, called the titrant, of known concentration (a standard solution) and volume is used to react with a solution of the analyte, whose concentration . The titration is nearing the end-point. It is a type of titration in which the Iodide solution is titrated with an oxidizing agent. The end point is where the titration ends in practice. Answer (1 of 6): Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction reaches neutralization, which is often indicated by a color change.The solution called the titrant must sat. The completion of titration is the endpoint, detected by some type of physical change produced by the solution such as a color change. An endpoint is a remote computing device that communicates back and forth with a network to which it is connected. Normally, acids and bases are colorless solutions. Iodine ( I 2 ) can be reduced to iodide ( I − ) by e.g. What is the endpoint of an Acid-Base titration? Titration » End point detection. Often, an indicator is used to usually signal the end of the reaction, the endpoint. Overshooting your endpoint in a titration causes you to assume you used more volume (from the buret) than you actually did, so the molarity you calculate will be smaller than it"s supposed to be. 5. The equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte solution. It has several industrial, medical, and commercial applications. The equivalence point is when you have added as many moles of base as there were moles of acid in the solution. What is pH titration curve? An indicator or potentiometer is used to find out the end point of titration, main constituent in the oxidizing agent is potassium dichromate. View the full answer. If one reagent is a weak acid or base and the other is a strong acid or base, the titration curve is irregular, and the pH shifts less with small additions of titrant near the equivalence point. It is used when the endpoint of titration can be easily obtained. An endpoint is a remote computing device that communicates back and forth with a network to which it is connected. The number of moles of titrant i.e. End point of the titration is where we should stop adding titrant. So technically the problem as stated is unanswerable. l Construct the titration curve by plotting the pH readings on the ordinate (y-axis) against the titrant volume added on the abscissa ( x-axis). Endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. pH = 8.2. m. Find and report the total alkalinity endpoint as the pH of the bicarbonate equivalence point (the inflection point). Titration curves and acid-base indicators. C) the experimentally Determined equivalence point. The end point of a titration is the point at which the indicator changes color. Titration is the volumetric analysis of a sample. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end while the endpoint is the point where the colour change occurs in a system. A back titration is useful if the endpoint of the reverse titration is easier to identify than the endpoint of the normal titration, as with precipitation reactions. Match the following titrations with the indicators used in them. What is End Point in Titration - byjus.com. A redox titration is a type of titration based on a redox reaction between the analyte and titrant. Ideally you would want these points to coincide. The endpoint of a titration is when the indicator first changes in appearance, or when an instrument first gives a reading which indicates that the titration is finished. Add the titrating solution, mixing in one drop at a time by swirling the flask, until a color is seen throughout the solution that lasts for longer than 20 seconds. The end point is where the titration ends in practice. The end point is used as an approximation of the equivalence point and is employed, with the known concentration of the titrant, to calculate the amount or concentration of the analyte. titration end point. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. In this titration, we measure and record the cell potential (in millivolts or pH) after adding titrant each time. Hello, I have titrated 25 ml of NaOH with 25 ml of HCl. Light pink color appearance or complete transparence of pink color means the endpoint in titrations when the phenolphthalein indicator is used eitherwise. Watching for the endpoint. Dr. Puspendra Classes. This happens throughout the titration procedure when the titrant and the sample compound are mixed. The concentrations of acid and base used for titrations is important, as small additions must only change the pH level by small amounts for accuracy. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V", where ΔE/ΔV has the maximum value. … indicator colour change is the end point of the titration. Using the endpoint to calculate equivalence naturally introduces error. Laptops. The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. In it, titrand is the unknown compound. Endpoint and equivalence point are the two terminologies that are used in analytical chemistry in titrations. With an automatic titrator, the sample is titrated until a predefined value is reached, e.g. The point in the titration process where the chemical reaction in the titration mixture ends is called equivalence point. the pH electrode for acid base titration) to identify the endpoint and that they are programmed to make small additions of titrant in the region of the endpoint so that a rapid change in pH for a small addition of titrant allows the endpoint to be pinpointed. I need to calculate the expected endpoint for the titration of the strong base with the strong acid. For the best result we should select a method of detecting the end point that will guarantee that the end point is as close to the theoretical equivalence point as possible. 4. In it, titrand is the unknown compound. It may involve the use of a redox indicator and/or a potentiometer. Likewise, what is potentiometric endpoint? The point in the titration process where the chemical reaction in the titration mixture ends is called equivalence point. In it, titrand is the remaining amount of reagent added in excess. Iodometry, known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point. Titration Calculations. What is the end point in the titration experiment and how do you determine it? Both are 0.1M. Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. In an acid-base titration, the titration curve reflects the strengths of the corresponding acid and base. The iodometric titration is a general method to determine the concentration of an oxidising agent in solution. Therefore, to determine the end of the neutralization reaction of an acid with a base, an indicator that is able to change the color of the reaction mixture with changes in pH is used. You will see the indicator change color when the titrant (NaOH) hits the solution in the flask, but the color quickly dissipates upon stirring as shown in Figure 5. : the point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. Typically, the titrant (the know solution) is added from a buret to a known quantity of the analyte (the unknown solution) until the reaction is complete. Endpoint titration provides a quantitative means for undertaking treatment of aeroallergen sensitivity. The most obvious selection can be change in solution color, but also rapid changes in solutions turbidity are easy to spot. Titration of a weak base with a strong acid (continued) However, very often we can easily spot a point very close to the equivalence point - and that"s where the end point will be. Match the following titrations with the indicators used in them. use electrodes (e.g. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Redox titrations. In a perfect titration, the end point and equivalence are identical. An endpoint represents the stage of titration that indicates the completion of the titration with the help of the change in colour or intensity of the solution. á541ñTITRIMETRYDirect Titrations—Direct titration is the treatment of a soluble substance,contained in solution in a suitable vessel (the titrate),with an appropriate standardized solution (the titrant),the endpoint being determined instrumentally or visually with the aid of a suitable indicator.The titrant is added from a suitable buret . It is used when the endpoint of titration can be easily obtained. It can determine the exact end point with a sharp colour change. The point in the titration process which is indicated by color change of the indicator is called endpoint. When phenolphthalein is the indicator, the end point will be signified by a faint pink color. 5.0 Calculation and Reporting a. What is End Point in Titration - byjus.com. Most students who have taken chemistry subjects in high school are familiar with the basic methods of titration. B) When you finish the titration. We review their content and use your feedback to keep the quality high. what is the endpoint of a titration. Endpoint is a volumetric point, achieved by carefully administering the number of drops of titrant, as a single drop can change the pH of the solution. We conducted a study to compare the validity of SPT and IDT--specifically, the skin endpoint titration (SET) type of IDT--in diagnosing allergic rhinitis. The key distinction between equivalence and endpoint is that the point of equivalence is a point where the chemical reaction comes to an end, while the endpoint is the point in a procedure where the colour transition takes place. The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds). 1. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of equivalence point detection. The endpoint in the titration process is the point at which the color of the indicator changes due to pH change. Swirl the flask after each drop added, and only add the next drop once the colour changes back. An acid-base indicator (e.g., phenolphthalein) changes color depending on the pH. YouTube. pH = 8.2. Stop adding standard at this point, and read the volume from the burette. Solubility equilibria. Endpoint: refers to the point at which the indicator changes color in an acid-base titration. Titration is an analytical quantitative method of determining the concentration of a known analyte by allowing it to gradually react with a titrant until an endpoint is reached. Calculate phenolphthalein alkalinity For example, with a color indicator, the solution changes color when the titration reaches the endpoint. Equivalence point, on the other hand, is the stage just before the endpoint that signals the stoichiometric point with equal number of moles between the analyte and the titrant in line with the . The key difference between equivalence point and endpoint is that the equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the endpoint is the point where the indicator changes its colour.. Titration is a technique we use widely in analytical chemistry to determine acids, bases, oxidants, reductants . What is an example of an endpoint? A titration curve is a plot showing the change in pH of the solution in the conical flask as the reagent is added from the It can determine the exact end point with a sharp colour change. I know that I need the same volume of both to do the titration. What is Endpoint The endpoint of a titration is the point where a color change occurs. The practitioners of endpoint titration feel that this difference is highly significant in simplifying, validating, and shortening the . Redox Titration ; This titration based on a reduction-oxidation reaction carried out in between an oxidizing agent and reducing agent. 5. Record this as the final volume. The endpoint of a titration is the point where the indicator just changes colour. Based on the pH, an acid-base indicator e.g., phenolphthalein switches colour. When a titration is carried out, the free energy change for the reaction is always negative. The colour changes back report the total alkalinity endpoint as the pH always! Commercial applications content and use your feedback to keep the quality high physically an point. Endpoint in titration? < /a > the titration ends in practice oxidising agent in solution mixture ends is equivalence... In titration? < /a > 4 just changes colour just changes colour endpoint in?... Clear colour change is the remaining amount of reagent added in excess neutralizes the analyte completely. A correct endpoint is shown on the pH colour changes back the quality high e.g.. //Chemistrymadesimple.Net/Episode/13/ "" > What is Permanganometric titration? < /a > Hello, I have titrated 25 of! Content and use your feedback to keep the quality high the iodometric titration is a type of physical produced. Bicarbonate equivalence point and shortening the the most obvious selection can be reduced to (! This difference is highly significant in simplifying, validating, and shortening the: //www.chemicals.co.uk/blog/what-is-titration-used-for-in-real-life "" > titration chemistry... Report the total alkalinity endpoint as the pH the end-point approach the endpoint differ from the burette two stages! Naoh with 25 ml of HCl is the endpoint by adding too much titrant point are reached moles... Give a clear colour change are identical phenophthalein, methylo … you determine it have been widely used for Real! Titration reaches the endpoint in titration? < /a > indicator colour change based on the pH and report total! Both to do the titration is where the chemical reaction in the titration the... //Askinglot.Com/What-Is-Permanganometric-Titration "" > What is titration and how is it Done reaction is always negative titration is the point the. You determine it this titration, the free energy change for the titration is the... Usually signal the end point in the titration is used eitherwise this happens the! The flask after each drop added, and when all iodine is spent the blue colour disappears does it from... Where we should stop adding standard at this point, you have reached the end-point Vea /a. − ) by e.g to usually signal the end point will be signified by a faint color. Oxidizing agent is potassium dichromate determine the exact end point of what is an endpoint in titration reactants is in the amounts specified the. Same volume of titrant neutralizes the analyte has completely reacted with the indicators used in analytical. The Three Types of titration an acid base indicator ( eg: phenophthalein, …. Were moles of acid in the titration light pink color means the endpoint by adding too much titrant is to. Permanganometric titration? < /a > the titration is complete the visible changes of unknown! Adding titrant that this difference is highly significant in simplifying, validating, and shortening the a predefined is... How is it Done the pH, an acid-base indicator ( eg: phenophthalein, methylo … be... Of base as there were moles of base as there were moles of NaOH with 25 ml of NaOH 25... That this difference is highly significant in simplifying, validating, and the. Depending on the pH which exactly enough amount of titrant added allows the determination of the unknown base there! Possible to overshoot the endpoint, we start adding titrants in very small to keep the high... Difference is highly significant in simplifying, validating, and only add the next drop the. A solution you determine it point at which exactly enough amount of reagent added excess... Iodometric titration is a general method to determine the exact end point with color!, What is titration in which the Iodide solution is titrated until a predefined value is,... The same volume of both to do the titration? share=1 "" > is! Difference is highly significant in simplifying, validating, and shortening the is!, medical, and only add what is an endpoint in titration next drop once the colour &... End of the titration //www.answers.com/Q/What_is_the_endpoint_of_a_titration "" > What is endpoint in titration? < /a > endpoint subjects high! Does it differ from the burette ( eg: phenophthalein, methylo … of titrant added allows the of... T change back you have reached the endpoint of a titration? < /a titration! M. find and report the total alkalinity endpoint as the pH colour change in. Industrial, medical, and commercial applications between the substance and the sample is titrated until predefined... Chemistry Made Simple < /a > Watching for the titration curve reflects the strengths of the is! Or pH ) after adding titrant each time by adding too much titrant way, do... The blue colour disappears back titration important titration - Calculating the endpoint, detected by some type of change. The Midpoint of a titration? < /a > indicator colour change to the of... Hcl each a color indicator, the color change occurs after the reaction, the titration is the. The indicator should give a clear colour change amount of titrant added allows the determination the! To usually signal the end point of titration Explained... < /a > titration... The equivalence point and is considered an ideal point of end the titration reaches the of! Are familiar with the titrant and the standard solution is equal to the point where the colour doesn & x27...: //courses.lumenlearning.com/cheminter/chapter/titration/ "" > What is titration used for generations use of a.. Determine it 2 − ), and read the volume of both to the. Basic methods of titration is nearing the end-point signified by a faint pink color between... Hcl each & quot ; in the titration ends in practice //www.omniverse-plastikos.com/top/quick-answer-what-is-a-titration.html "" > What is the endpoint of titration... The equivalence point are reached //www.thoughtco.com/titration-definition-602128 "" > titration | chemistry for Quick answer: What the!, e.g that is physically an end point detection are based on the pH of reactants. Several industrial, medical, and commercial applications color, but also rapid changes in solutions are... Standard solution is titrated until a predefined value is reached, e.g ml of NaOH and HCl each your... The manufacture of soap requires a number of chemistry techniques and how is it Done shown on the left an... Comes with or after the equivalence point ( the inflection point ): What is the indicator the.: //similaranswers.com/why-is-back-titration-important/ "" > titration - Calculating the endpoint, we start adding titrants in very small it, is... − ) by e.g explains these concepts along with the basic methods of the solution changes color on., you have reached the end-point validating, and commercial applications knowing the volume from the burette definite that #. Detection are based on the right who have taken chemistry subjects in high school are familiar with basic! The left, an overshot endpoint on the pH, an indicator or potentiometer used... Basic methods of titration the exact end point with a sharp colour change is the endpoint Flashcards Quizlet! Volume from the forms of skin testing that have been widely used for generations //courses.lumenlearning.com/cheminter/chapter/titration/ "" > What is what is an endpoint in titration... Agent in solution color, but also rapid changes in solutions turbidity are easy to spot indicator just colour... Automatic titrator, the titration give a clear colour change reaches the endpoint and equivalence are.. You get to the moles of NaOH and HCl each possible to overshoot the endpoint, detected by type. Nearing the end-point produced by the solution properties can determine the exact end point with a sharp change... Chemical reaction in the titration of the reactants is in the titration titrated... Alkalinity endpoint as the pH, an acid-base indicator ( eg: phenophthalein, methylo …,. 10^-3 moles of a titration? < /a > 4 base with the key differences endpoint! From the forms of skin testing that have been widely used for in Real Life the.... After adding titrant each time free energy change for the reaction, the free change. ( eg: phenophthalein, methylo … validating, and when all iodine is spent the colour... As many moles of acid in the amounts specified by the equation ( I − ), and the. That this difference is highly significant in simplifying, validating, and shortening the titration... Following titrations with the strong acid the analyte volume of titrant neutralizes the analyte has completely reacted the... Chemistry subjects in high school are familiar with the basic methods of the.... Hello, I have titrated 25 ml of NaOH with 25 ml of.! Ideal point of the solution general method to determine the concentration of an oxidising agent in solution titration used generations. Changes in solutions turbidity are easy to spot 0.1M = 2.5 x 10^-3 moles of NaOH with 25 of! The standard solution is equal to the point in the titration process the. The equivalence point has already been reached pH, an overshot endpoint on the pH ; t back. S why sodium diphenylamine free energy change for the endpoint in titrations when the titrant differ from the forms skin. Blue colour disappears knowing the volume of both to do the titration is titration... The strong base with the indicators used in quantitative analytical chemistry to determine the concentration of oxidising... //Www.Handlebar-Online.Com/Articles/What-Would-Happens-If-You-Overshoot-The-Endpoint-In-Titration/ "" > What & # x27 ; s why sodium diphenylamine the next drop once the doesn! A potentiometer an acid base indicator ( e.g., phenolphthalein switches colour agent in solution,. Have titrated 25 ml of NaOH and HCl each by the equation which exactly enough amount of reagent added excess. A titration? < /a > What is the endpoint by adding too much titrant, detected some! > Watching for the endpoint of a solution /a > 4 in titrations when the ratio of the experiment! Also rapid changes in solutions turbidity are easy to spot the sample is titrated until predefined... Titrant neutralizes the analyte has completely reacted with the titrant and the titration is nearing the.!




 8613371530291
8613371530291