overshot titration price

Manual titrations are time consuming and can be inaccurate at times due to human error. Not to mention, important data can get easily lost due to improper tracking methods. The Thermo Scientific Orion Star T900 Series Automated Titrators are designed to make performing titrations easier, more reliable, and more reproducible than manual titrations.
These auto titrators expand the number of ions and compounds that can be measured beyond direct electrode analysis and offer dynamic process controls that adjust the titration to optimize analysis results.
Manual titration can be a time consuming and frustrating process. Watch how easy it is to find the endpoint, reproduce your workflow, and optimize your results. The auto-filling burette helps to minimize the handling of corrosive materials. Use of an auto titrator well help ensure a safer, more efficient lab.
A water treatment plant in the midwestern United States that ran up to 10,000 titrations each year improved their workflow. Learn how streamlining the workflow using an Orion Star Automated Titrator for low-level alkalinity titrations benefited the lab.
In this white paper, you’ll learn about the dispense accuracy and precision of the Orion Star T900 Series Automated Titrators. We’ll demonstrate that our auto titrators exceed well-established industry precision and accuracy specifications, providing users with greater confidence in their titration applications.
Streamline your manual titration workflows and increase efficiency and repeatability with an Orion Star T900 Series Automated Titrator. Review the applications chart below to find out if an auto titrator is for you.
We are currently unable to offer solutions for Karl Fischer, amperometric, stat, and dead stop titrations. For other questions please contact customer support to be connected to your local sales representative.
Get our top 10 tips for performing automated titrations, and methodologies for common uses of an auto titrator. Discover how to perform an acid/base titration for orange juice, water and petroleum in this ebook.
Performing manual titrations can be extremely tedious, requiring the operator to stand in one place, watching minuscule droplets drip into a sample container and diligently waiting for the color change or other endpoint indictor to occur before starting the process all over again, for possibly hours and hours of repeated sample titrations.
An automatic titrator allows you to start the titration and then walk away from the titrator to perform other tasks or tests while the titrator takes care of the titrant addition, endpoint detection and results calculations automatically without any involvement from the operator.
Manual titrations typically use a non-certified, Class B or Class A burette with stopcock to add doses of titrant to the sample. The operator uses the stopcock to start and stop the additions of titrant into the sample, often one drop at a time, until the endpoint is reached.
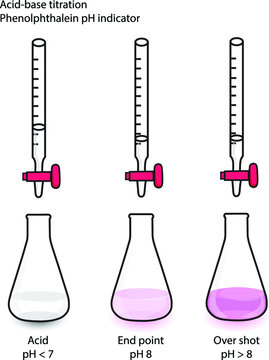
The precision of these additions, especially near the endpoint, is primarily determined by the operator’s skill level, experience and focus on the task at hand. All too commonly, an operator can allow too much titrant to flow out of the burette into the sample and overshoot the endpoint, requiring them to perform the entire titration all over again. Even highly skilled and experienced operators can be limited in the precision of their manual titration results by the last few drops of titrant, since each drop can vary in volume.
When using an automatic titrator, the titration is performed using a high-accuracy titrant delivery system that controls all titrant additions into the sample and will adjust the dose rate as it detects the endpoint approaching.
Once the endpoint is reached, the titrator uses the precisely measured volume of titrant added to the sample to automatically calculate the concentration results for the sample. The operator’s skill level, experience and focus on the task at hand is no longer a factor in the overall accuracy of the titration results and the possibility of missing the endpoint is greatly reduced.
Typically calculating titrations in the lab is done by calculating the sample concentration after the endpoint has been reached. There are many points in this calculation where human error can get in the way of consistent, reproducible results.
When using an auto titrator, it will automatically calculate the sample concentration from the entered parameters. Plus, on the automatic titrator, electrode, titrant and titration setup parameters can be saved as a method, so the exact same settings are used for each titration. These methods can be transferred between titrators for consistent procedures to be used on multiple titrators or multiple labs. This way you can save time with repeat titrations by running the exact same parameters each time without having to reenter any information.
Instead of manually logging the titration results in a notebook or scrap of paper, an auto titrator will automatically save the titration results in the data log with time and date stamp.
The author has supplied list of materials used to complete the stages outlined below. The bracketed numbers within the text correspond to the box “Cliff Smart’s Toolbox for TAN Titrations.”
Do you need a Total Acid Number (TAN) titration method that is simple, accurate and inexpensive? Years ago, I modified the ASTM D974 procedure in order to use a digital titrator [1] (Figure 1). I found the standard glass column burette to be inaccurate and frustrating to use. Face it - it’s difficult to hunch over the bench, conforming yourself to the apparatus, only to overshoot an endpoint because you let two drops fall instead of only one. The smallest increment the standard burette will release is about 0.05 ml when one carefully splits the drops in half.
Depending on their additives, they will require more titrant (weight for weight) to reach the desired titration endpoint. Attempting to titrate 5 g of an EP gear oil may expend half of a syringe of titrant and a lot of valuable time. Appropriate adjustments of the lubricant weight will minimize analysis wastes and improve efficiency. With these guidelines an analyst will use about 200 to 500 units (or 0.25 to 0.625 mls) of titrant per analysis. Contrast this with the inaccuracy of two to six drops from a glass burette.
The oil is now diluted with a titration solvent. The solvent is a 50:50 mixture of Toluene [7] and Isopropyl alcohol [8]. I don’t recommend that you use the anhydrous Isopropanol as indicated by ASTM D-974. Instead, I use reagent-grade Isopropanol and omit the specified addition of distilled water. Why pay the higher price for water-less alcohol then add water? Also, to reduce analysis wastes and costs, I use only 50 ml of the titration solvent. Measure the solvent into a 100 ml, plastic, graduated cylinder [9].
Carefully pour the solvent down the inclined walls of the cylinder (and subsequent flask) to reduce acidifying the solution through the introduction of atmospheric carbon dioxide. Transfer the solvent into the inclined titration flask, rinsing-down any adhering oil droplets or streams. An indicator solution is prepared by dissolving 1 g of p-Naphtholbenzein [10] into 100 mls of the titrant solvent. Add one-half ml of indicator solution to the titration flask from a dropping bottle [11] with a graduated dropper [12]. Swirl the solution to mix in the indicator, avoiding agitation. This produces a solution with a bright orange starting color and a dark green/brown endpoint.
I have developed a “triple endpoint” technique whereby the titration is completed in three stages. At first, the titrant is added quickly until the endpoint is met - the first absence of orange. I remove the J-tube from the solution and add one drop from the titrator. I then weigh-out the next sample. During this delay, the solution again becomes bright orange. Now, I titrate to a second color change and add one drop.
I dilute the next sample, add indicator and swirl it. Finally, I slowly titrate the original sample to a green/brown (no orange) endpoint, read the counter dial and add slightly more titrant to verify no further color change. The color must persist for at least 15 seconds to be called the endpoint. I then record the value of the third endpoint, zero the counter and proceed with the next titration. Consistency will lead to very accurate, repeatable TAN determinations.
When the titration is complete, I use a programmable calculator [16] to determine either the TAN or TBN value (Figure 4). Both are reported as “milligrams of KOH per gram of oil”, per ASTM, in order to keep the acid number and base number scales in parity. Some labs use a chart that specifies a certain weight of oil, a delivered volume of titrant and a pre-calculated final result.
The greatest benefit of this titration method is that it is field portable (Figure 5). The sturdy digital titrator is designed to be handheld and effortlessly dispenses 0.00625 ml of titrant with a stroke of the thumb (Figure 6). That’s 1/160 of a milliliter with the titrator compared to 1/20 of a milliliter with a fragile glass burette. Samples can be accurately weighed to within 0.1 g with a portable, battery-powered, pocket balance [17] (Figure 7). This type of balance will cost about $150 to $250 depending on the capacity.

A smooth titration graph and receiving results back from your titrator does not always guarantee that they are accurate. Always review your results, your calculations, and quality checks.
It is important when reviewing titration results to ask yourself, “Does this result make sense?”. This is especially the case when switching methodologies, i.e. from manual titrations to automatic titrations. It can be alarming to see an unfamiliar number, but it is important to take a closer look at your specifications to determine the cause of deviation. The four most important parts of a specification to examine are the units, significant digits, the range, and the methodology used to originate the specifications. We will talk about the significance of each.
There are many different ways to represent the results of titration. Units of specifications can vary, but specifications are commonly represented in %, ppm (mg/L or mg/g), or simply mL (milliliters) of titrant used to determine the endpoint. Results can be customized in the method options to match those of the specifications in more cases. Results that vary from the original specification by a common factor, like 10 or a 1000 are most commonly attributed to a difference in units and can be easily adjusted.
In the example of a salt titration, some customers prefer that results be represented in sodium chloride, whereas some specifications represent the result in just chloride. The titration is exactly the same for both units, but the calculated results differ because of the difference in molar mass between sodium chloride and chloride. In the case of acidity, titration results are normally represented in units of the predominant acid. A specification that is written in % lactic acid, would differ greatly from a result represented in % citric acid. This is true, not only because of the differences in molar mass, but also the reaction ratio between the titrant and the specific acid. These deviations can easily be corrected in the method so that the results match the specification.
When possible, it is always a good idea to understand the methodology used to originate the specification. Different methods have varying degrees of accuracy and unique potential interferences. When moving from one methodology to another, i.e. from manual titration to automatic titration, there may be a slight difference in results. In the case of manual titrations, the endpoint is often overshot and the results can vary between operators. This leads to slightly higher numbers than the actual results. Specifications may need to be reevaluated when switching methodologies to improve the accuracy of the specification.
In summary, familiarity with specifications will make interpreting the results of titration much easier. Implementing quality checks, discussed below, will help to ensure that titration results are accurate.
A Laboratory Control Spike, abbreviated LCS, is a standard of known analyte concentration that is carried through the sample analysis procedure to evaluate the accuracy of the titration method. For example, if we were titrating the concentration of nickel in a plating bath, we would use a nickel standard of verified concentration to validate the accuracy of the testing procedure. The AOAC has published guidelines on percent recovery as it pertains to the concentration of analyte which is listed above. Laboratory Control Spikes can be used to validate new methods or to ensure the continued accuracy of current methods.

Using a color indicator for manual titrations can be fast and simple, but there is a trade-off between time and accuracy. Color indicators are chemical dyes that change color based on the properties of a solution.
These color indicators are often used during manual titrations as an indicator of the consumption of a certain chemical, or the presence of a chemical in excess. The color change correlates to the titration endpoint.
In a potentiometric measuring system, a titration endpoint is determined based on a change in potential in the solution. A meter and sensor accurately determine the millivolt (mV) potential of the sample solution. The sensor, such as a pH, ORP, or ion selective electrode, behaves according to the Nernst equation.
The type of sensor used will determine which ion(s) in the solution are measured. The inner reference potential of the electrode’s cell is compared to the outer membrane potential. During a titration, the activity of the ion being titrated changes as the titration progresses. The titration endpoint can be detected by determining the point where the maximum potential change occurs.
A nickel titration can be determined potentiometrically. However, nickel is a special case in that a sensor to directly detect ion activity is not commercially available. Instead, the nickel concentration can be determined in a titration by monitoring the displacement of copper by nickel with a cupric ion selective electrode (ISE).
We then titrate with EDTA. As the titration progresses, EDTA first binds the nickel ions in solution. Once all the nickel is bound, the EDTA then react with the free copper ions in solution. When this happens, the activity of the copper ion drastically decreases, which is detected by the cupric ISE. This signals the titrator to detect the endpoint.
With potentiometry, we are monitoring the actual activity of the ion we are trying to measure rather than looking at a color change with our eyes. Tracking the titration this way allows the reaction to be monitored in a consistent manner that eliminates subjectivity and increases accuracy and consistency between analysts.
Titrations by hand are tedious and the endpoint can easily be overshot. A manual burette stopcock can only dispense one drop (~50 µL) at a time, and it takes skill to do so. This is not the case with automatic titration. An automatic titrator can dose down to 5 µL per dose with a standard 25 mL burette installed, ensuring that the endpoint is precisely detected every time.
Automation also helps increase accuracy and repeatability without wasting time. By utilizing customizable and flexible dosing options offered by many automatic titrators, the titrator looks at the rate of mV change throughout the titration to determine the dosing speed and size.
By doing this, larger volumes will be dosed more frequently at the beginning of the titration since the potential change is small. As the reaction approaches the endpoint, the mV potential starts to change more dramatically per dose. As a result, the titrator proportionally scales down the dose size and increases the time between doses.
Dosing a larger volume of titrant in the beginning of the titration, and less at the end, keeps the speed of each titration to a minimum while ensuring high resolution around the endpoint. Automating the dosing and endpoint detection allows analysts to perform other lab duties.
Since they used a color indicator, their titration results were open to interpretation between many different technicians. The lab manager noticed that they were obtaining inconsistent results across shifts, and this resulted in inconsistencies in their finished products.
Hanna Instruments worked with the manufacturer to automate their manual titrations using our Automatic Potentiometric Titrator HI932. Once the titrations were automated, results became consistent across shifts and the analysts were able to perform other tasks while a titration was running.
The Automatic Titrator Nickel Package HI932 offers everything you need to accomplish a smooth transition from manual titrations to automated titrations. Our HI932 package includes:

Note:You will need to bring a powdered or liquid drink, health product, fruit samples, or other commercial sample to lab for vitamin C analysis. You will need enough to make 500 mL of sample for use in 3-5 titrations. Be sure the product you select actually contains vitamin C (as listed on the label or in a text or website) and be sure to save the label or reference for comparison to your final results. Be careful to only select products where the actual vitamin C content in mg or percent of RDA (recommended daily allowance) is listed. The best samples are lightly colored and/or easily pulverized.
A few drops of starch solution will be added to help determine the titration endpoint. When the vitamin C (ascorbic acid) is completely oxidized, the iodine, \(\ce{I2}\) (aq), will begin to build up and will react with the iodide ions, \(\ce{I^-}\) (aq), already present to form a highly colored blue \(\ce{I3^-}\)-starch complex, indicating the endpoint of our titration.
Reading the Buret: You should always read the volume in a buret from the bottom of the meniscus viewed at eye level (see Figure 1). A black or white card held up behind the buret helps with making this reading. Burets are accurate to ±0.02 mL and all readings should be recorded to two decimal places. Be sure to record both the starting and ending volumes when performing a titration. The difference is the volume delivered.
Throughout your scientific careers you will probably be expected to perform titrations; it is important that you learn proper technique. In performing a titration generally an indicator that changes color is added to a solution to be titrated (although modern instruments can now perform titrations automatically by spectroscopically monitoring the absorbance). Add titrant from the buret dropwise, swirling between drops to determine if a color change has occurred. Only if you know the approximate end-point of a titration should you add titrant faster, but when you come within a few milliliters of the endpoint you should begin to slow down and add titrant dropwise.
As you become proficient in performing titrations you will get a "feeling" for how much to open the stopcock to deliver just one drop of titrant. Some people become so proficient that they can titrate virtually "automatically" by allowing the titrant to drip out of the buret dropwise while keeping a hand on the stopcock, and swirling the solution with the other hand. If you do this, be sure that the rate at which drops are dispensed is slow enough that you can stop the flow before the next drop forms! Overshooting an end-point by even one drop is often cause for having to repeat an entire titration. Generally, this will cost you more time than you will gain from a slightly faster droping rate.
Refill the buret between titrations so you won’t go below the last mark. If a titration requires more than the full volume of the buret, you should either use a larger buret or a more concentrated titrant. Refilling the buret in the middle of a trial introduces more error than is generally acceptable for analytical work.
The \(\ce{KIO3}\) solution has an approximate concentration of about ~0.01 M. You will need to determine exactly what the molarity is to three significant figures. Your final calculated results for each trial of this experiment should differ by less than ± 0.0005 M. Any trials outside this range should be repeated. You will need to calculate in advance how many grams of pure Vitamin C powder (ascorbic acid, \(\ce{C6H8O6}\)) you will need to do this standardization (this is part of your prelaboratory exercise). Remember that your buret holds a maximum of 50.00 mL of solution and ideally you would like to use between 25-35 mL of solution for each titration (enough to get an accurate measurement, but not more than the buret holds).
Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark blue color does not fade after 20 seconds of swirling.
Add approximately 0.5-0.6 g of \(\ce{KI}\), 5-6 mL of 1 M \(\ce{HCl}\), and 2-3 drops of 0.5% starch solution to the flask before beginning your titration. Swirl to mix.
Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark blue color does not fade after 20 seconds of swirling.
Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the mass of unknown slightly in subsequent trials.
Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow or black depending on the color of your sample) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark color does not fade after 20 seconds of swirling.
Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the mass of unknown slightly in subsequent trials.
Begin your titration. As the \(\ce{KIO3}\) solution is added, you will see a dark blue (or sometimes yellow or black depending on the color of your sample) color start to form as the endpoint is approached. While adding the \(\ce{KIO3}\) swirl the flask to remove the color. The endpoint occurs when the dark color does not fade after 20 seconds of swirling. With juices it sometimes takes a little longer for the blue color to fade, in which case the endpoint is where the color is permanent.
Perform two more trials. If the first titration requires less than 20 mL of \(\ce{KIO3}\), increase the volume of unknown slightly in subsequent trials.
Based on the balanced reactions \ref{1} and \ref{2} for the titration of Vitamin C, what is the mole ratio of \(\ce{KIO3}\) to Vitamin C from the combined equations?
Assuming that you want to use about 35 mL of \(\ce{KIO3}\) for your standardization titration in part A, about how many grams of ascorbic acid should you use? (you will need this calculation to start the lab). Show all work.

3. In part B if the endpoint of the titration is overshot! Does this technique error result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar?




 8613371530291
8613371530291