dc rongsheng llc fishers supplier
We obtained bone marrow-derived dendritic cells (BMDCs) from BM suspensions prepared from mouse femurs as described previously (16). Exosomes were purified from the supernatants of DCs infected with T. gondii by differential centrifugation as previously described (17). Briefly, we harvested supernatants of DCs infected with T. gondii from the DC-Me49-exo group, and those of control DCs from the DC-exo group. The different supernatants were centrifuged at 500 g for 10 min to remove cell debris and other small particles, and the supernatants was collected. Then, we centrifuged the supernatant at 16,500 g for 20 min, followed by filtration through a 0.22-μm filter (Millipore Sigma). Finally, supernatant solutions were ultracentrifuged at 120,000 g for 90 min, and the exosome pellet was resuspended with an appropriate amount of PBS. We measured the protein content of exosomes using a BCA protein assay kit (Thermo Fisher, USA). Exosomes were stored at −80°C for future use or directly used in co-culture experiments.
Mouse BM–derived MDSCs were obtained as previously described (18, 19). In brief, we flushed BM cells from the femurs and tibias of approximately 5-week-old BALB/c mice. Red blood cells (RBCs) were lysed with lysis buffer (Thermo Fisher). The BM cells were then cultured in RPMI-1640 supplemented with 10% FBS, 1% penicillin-streptomycin solution and stimulated with 40 ng/mL interleukin-6 (IL-6) and granulocyte-macrophage colony stimulating factor (GM-CSF) (Rocky Hill, NJ, USA) at 37°C for 4 d in a 5% CO2-humidified atmosphere. Then, we detected unique M-MDSCs and G-MDSCs using (FITC)-CD45, (APC)-CD11b, (PE)-Ly6G, and (APC-700)-Ly6C. Uniquely DCs and macrophages were detected by (FITC)-CD45, (PC5.5)-CD11c, and (APC)-CD11b, (PB450)-F4/80. All the antibodies were purchased from Becton, Dickinson (San Jose, CA, USA). After induction and maturation, MDSCs were harvested. Then, MDSCs incubated with exosomes of DC-exo, DC-Me49-exo, and PBS, respectively. After 24 h, MDSC was collected for flow cytometry and Western blot. All cells were lysed using RIPA buffer (Beyotime Institute of Biotechnology, China) with 1 nM PMSF, and total protein concentration was determined using a BCA protein quantification kit (Thermo Fisher). Next, we loaded 5 µg total proteins per lane and resolved them on 0.6–0.8% gels by SDS-PAGE. Proteins were transferred onto PVDF membranes and blocked for 1 h with 5% non-fat milk at RT, after which the membranes were washed three times with PBST. Membranes were incubated for 1 h with monoclonal antibodies of P-JAK2, JAK2, P-STAT3, STAT3 and tubulin in a buffer containing PBST and 1% milk. All primary antibodies were purchased from CST. After washing them with PBST, we incubated membranes for 1 h with goat anti-mouse IgG-HRP (Santa Cruz, CA, USA) and detected signals on a ChemiDoc Touch Imaging (Bio-Rad, USA).
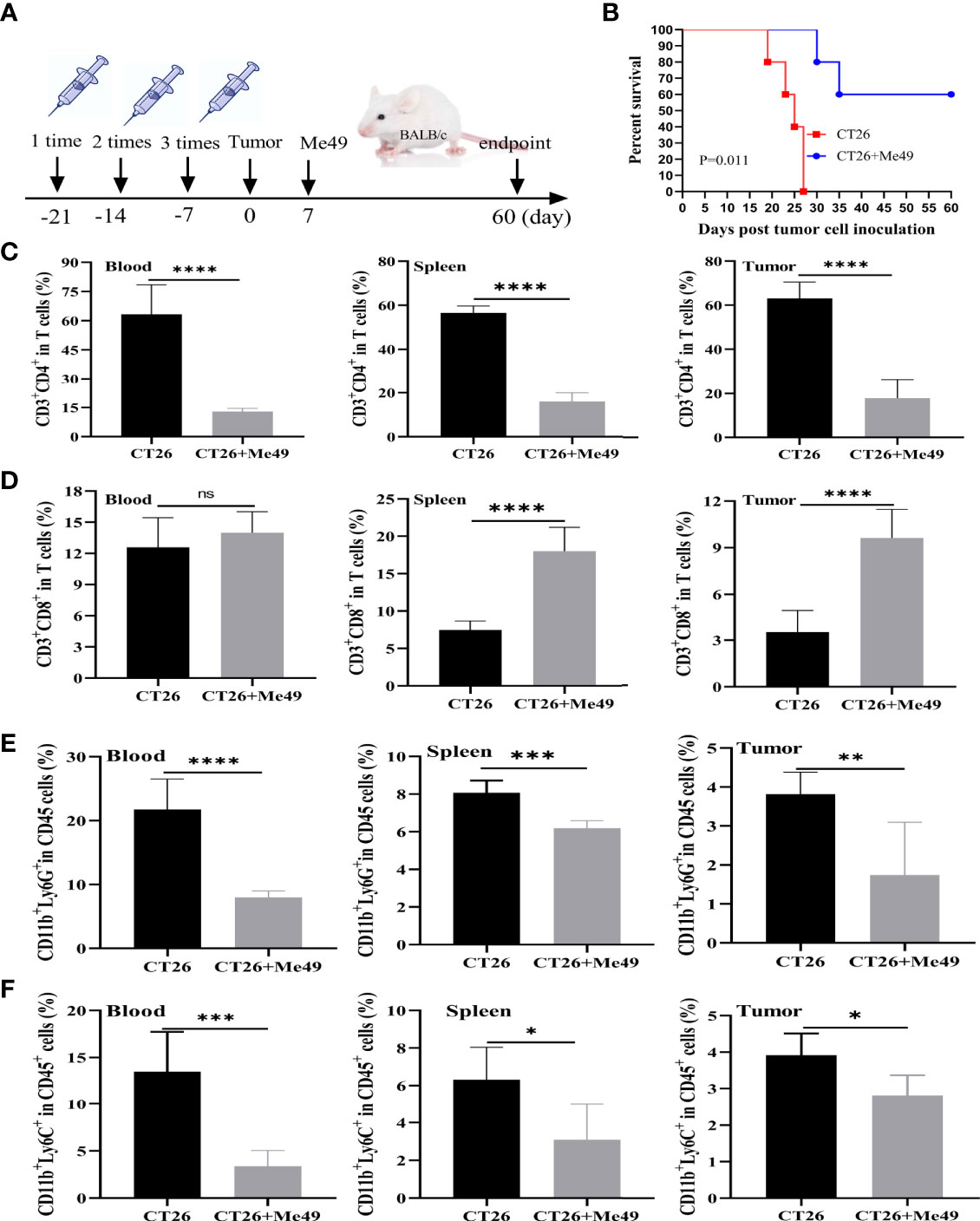
BALB/c female mice (6 weeks old) were weighed, randomly divided into three groups (n = 10 each group), and injected subcutaneously with 5 × 106 CT26 cells. When the tumor was visible, treatment was carried out according to the following design: an intra-tumoral injection with 10 μg DC-exo (DC-exo group), 10 μg DC-Me49-exo (DC-Me49-exo group), and 10 μL PBS (PBS group). Two injections were performed on day 1 and 3 after tumor visualization (8). At day 19 post inoculation, the tumor progression was monitored by using an IVIS Spectrum imaging system (IVIS Spectrum, USA). Mice were sacrificed, and blood, spleens and tumors were collected for flow-cytometry analysis. Cytokines in serum were determined by using IL-6 and GM-CSF ELISA kits (Neobioscience Technology Company).




 8613371530291
8613371530291