rongsheng jin in stock

Jahid S, Ortega JA, Vuong LM, Acquistapace IM, Hachey SJ, Flesher JL, La Serra MA, Brindani N, La Sala G, Manigrasso J, Arencibia JM, Bertozzi SM, Summa M, Bertorelli R, Armirotti A, Jin R, Liu Z, Chen CF, Edwards R, Hughes CCW, De Vivo M, Ganesan AK. PMID: 35385746; PMCID: PMC9127750.
Chen P, Zeng J, Liu Z, Thaker H, Wang S, Tian S, Zhang J, Tao L, Gutierrez CB, Xing L, Gerhard R, Huang L, Dong M, Jin R. PMID: 34145250; PMCID: PMC8213806.
Chen P, Lam KH, Liu Z, Mindlin FA, Chen B, Gutierrez CB, Huang L, Zhang Y, Hamza T, Feng H, Matsui T, Bowen ME, Perry K, Jin R. PMID: 31308519; PMCID: PMC6684407.

Lam, K. H., Guo, Z., Krez, N., Matsui, T., Perry, K., Weisemann, J., Rummel, A., Bowen, M. E. & Jin, R. A viral-fusion-peptide-like molecular switch drives membrane insertion of botulinum neurotoxin A1. Nat Commun 9, 5367 (2018) doi: 10.1038/s41467-018-07789-4.
Chen, P., Tao, L., Liu, Z., Dong, M. & Jin, R. Structural insight into Wnt signaling inhibition by Clostridium difficile toxin B. FEBS J (2018) doi: 10.1111/febs.14681.
Chen, P., Tao, L., Wang, T., Zhang, J., He, A., Lam, K. H., Liu, Z., He, X., Perry, K., Dong, M*. & Jin, R*. Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B. Science 360, 664-669 (2018) (*corresponding authors) doi: 10.1126/science.aar1999. PMCID: PMC6231499
Lam, K. H., Sikorra, S., Weisemann, J., Maatsch, H., Perry, K., Rummel, A., Binz, T. & Jin, R. Structural and biochemical characterization of the protease domain of the mosaic botulinum neurotoxin type HA. Pathog Dis 76 (2018) doi: 10.1093/femspd/fty044. PMCID: PMC5961070
Silva, D. A., Stewart, L., Lam, K. H., Jin, R. & Baker, D. Structures and disulfide cross-linking of de novo designed therapeutic mini-proteins. FEBS J 285, 1783-1785 (2018) doi: 10.1111/febs.14394. PMCID: PMC6001749
Lam, K. H., Qi, R., Liu, S., Kroh, A., Yao, G., Perry, K., Rummel, A. & Jin, R. The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein. Toxicon 147, 19-26 (2018) doi: 10.1016/j.toxicon.2017.10.012. PMCID: PMC5902665
Chevalier, A., Silva, D.A., Rocklin, G.J., Hicks, D.R., Vergara, R., Murapa, P., Bernard, S.M., Zhang, L., Lam, K.H., Yao, G., Bahl, C.D., Miyashita, S.I., Goreshnik, I., Fuller, J.T., Koday, M.T., Jenkins, C.M., Colvin, T., Carter, L., Bohn, A., Bryan, C.M., Fernández-Velasco, D.A., Stewart, L., Dong, M., Huang, X., Jin, R., Wilson, I.A., Fuller, D.H. & Baker, D. Massively parallel de novo protein design for targeted therapeutics. Nature 550(7674):74-79 (2017) doi: 10.1038/nature23912. PMCID: PMC5802399
Yao, G., Lam, K.H., Weisemann, J., Peng, L., Krez, N., Perry, K., Shoemaker, C.B., Dong, M., Rummel, A. & Jin, R. A camelid single-domain antibody neutralizes botulinum neurotoxin A by blocking host receptor binding. Sci Rep. 7;7(1):7438. (2017) doi: 10.1038/s41598-017-07457-5. PMCID: PMC5547058
Yao, G., Lam, K.H., Perry, K., Weisemann, J., Rummel, A. & Jin, R. Crystal Structure of the Receptor-Binding Domain of Botulinum Neurotoxin Type HA, Also Known as Type FA or H. Toxins (Basel) 9, 93 (2017) doi: 10.3390/toxins9030093. PMCID: PMC5371848
Yao, G., Zhang, S., Mahrhold, S., Lam, K. H., Stern, D., Bagramyan, K., Perry, K., Kalkum, M., Rummel, A.*, Dong, M.* & Jin, R.* N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat Struct Mol Biol 23 (7):656-662 (2016) (*corresponding authors) doi: 10.1038/nsmb.3245. PMCID: PMC5033645
Lee, K., Lam, K. H., Kruel, A. M., Mahrhold, S., Perry, K., Cheng, L. W., Rummel, A. & Jin, R. Inhibiting oral intoxication of botulinum neurotoxin A complex by carbohydrate receptor mimics. Toxicon 107, 43-49 (2015) doi: 10.1016/j.toxicon.2015.08.003. PMCID: PMC4658216
Lam, K.H. & Jin, R. Architecture of the botulinum neurotoxin complex: a molecular machine for protection and delivery. Current Opinion in Structural Biology 31:89-95 (2015) doi: 10.1016/j.sbi.2015.03.013. PMCID: PMC4476938
Lam, K.H., Yao, G. & Jin, R. Diverse binding modes, same goal: The receptor recognition mechanism of botulinum neurotoxin. Progress in Biophysics and Molecular Biology 117(2-3):225-31 (2015) doi: 10.1016/j.pbiomolbio.2015.02.004. PMCID: PMC4417461
Lam, T.I., Stanker, L.H., Lee, K., Jin, R. & Cheng, L.W. Translocation of botulinum neurotoxin serotype A and associated proteins across the intestinal epithelia. Cellular Microbiology 17(8):1133-1143 (2015) doi: 10.1111/cmi.12424. PMCID: PMC4610714
Matsui, T.*, Gu, S., Lam, K.H., Carter, L.G., Rummel, A., Mathews, II. & Jin, R.* Structural Basis of the pH-Dependent Assembly of a Botulinum Neurotoxin Complex. J. Mol. Biol. 426(22):3773-3782 (2014) doi: 10.1016/j.jmb.2014.09.009. (*corresponding authors) PMCID: PMC4252799
Lee, K., Zhong, X., Gu, S., Kruel, A.M., Dorner, M.B., Perry, K., Rummel, A., Dong, M. & Jin, R. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science 344(6190):1405-1410 (2014) doi: 10.1126/science.1253823. PMCID: PMC4164303
Lee, K., Lam, K.H., Kruel, A.M., Perry, K., Rummel, A. and Jin, R. High-resolution crystal structure of HA33 of botulinum neurotoxin type B progenitor toxin complex. Biochem. Biophys. Res. Commun. 446(2):568-573 (2014) doi: 10.1016/j.bbrc.2014.03.008. PMCID: PMC4020412
Yao, Y., Lee, K., Gu, S., Lam, K.H. & Jin, R. Botulinum Neurotoxin A Complex Recognizes Host Carbohydrates through Its Hemagglutinin Component, Toxins (Basel) 6(2):624-635 (2014) doi: 10.3390/toxins6020624. PMCID: PMC3942755
Lee, K., Gu, S., Jin, L., Le, T.T.N., Cheng, L.W., Strotmeier, J., Kruel, A.M., Yao, G., Perry, K., Rummel, A.* & Jin, R.* Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLoS Pathog. 9(10): e1003690 (2013) doi:10.1371/journal.ppat.1003690. (*corresponding authors) PMCID: PMC3795040
Zong, Y. and Jin, R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 70(17):3077-88 (2013) doi: 10.1007/s00018-012-1209-9. PMCID: PMC4627850
Gu, S. and Jin, R. Assembly and function of the botulinum neurotoxin progenitor complex. Curr. Top. Microbiol. Immunol. 364:21-44 (2013) doi: 10.1007/978-3-642-33570-9_2. PMCID: PMC3875173
Gu, S., Rumpel, S., Zhou, J., Strotmeier, J., Bigalke, H., Perry, K., Shoemaker, C.B., Rummel, A. & Jin, R. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science 335(6071):977-81 (2012) doi: 10.1126/science.1214270. PMCID: PMC3545708
Zong, Y., Zhang, B., Gu, S., Lee, K., Zhou, J., Yao, G., Figueiredo, D., Perry, K., Mei, L.* & Jin, R.* Structural basis of neuron-specific regulation of postsynaptic differentiation. Gene & Development 26:247-258 (2012) doi: 10.1101/gad.180885.111. (*corresponding authors) PMCID: PMC3278892
Yao, G., Zong, Y., Gu, S., Zhou, J., Xu, H., Mathews, II. & Jin, R. Crystal structure of the glutamate receptor GluA1 amino-terminal domain. Biochem. J. 438(2):255-63 (2011) doi: 10.1042/BJ20110801. PMCID: PMC3296483
Strotmeier, J., Gu, S., Jutzi, S., Mahrhold, S., Zhou, J., Pich, A., Eichner, T., Bigalke, H., Rummel, A.*, Jin, R.* & Binz, T*. The biological activity of botulinum neurotoxin type C is dependent upon novel types of ganglioside binding sites. Mol. Microbiol. 81(1):143-56 (2011) doi: 10.1111/j.1365-2958.2011.07682.x. Epub 2011 Jun 2. (*corresponding authors)
Strotmeier, J., Lee, K., Völker, A.K., Mahrhold, S., Zong, Y., Zeiser, J., Zhou, J., Pich, A., Bigalke, H., Binz, T., Rummel, A.* & Jin, R.* Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 431(2):207-16 (2010) (*corresponding authors)
Jin, R.*, Singh, S.K., Gu, S., Furukawa, H., Sobolevsky, A.I., Zhou, J., Jin, Y. & Gouaux E.* Crystal structure and association behavior of the GluR2 amino-terminal domain. EMBO J. 28(12):1812-23 (2009) (*corresponding authors) PMCID: PMC2699365
Kumar, J., Schuck. P., Jin, R. & Mayer, M.L. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat. Struct. Mol. Biol. 16(6):631-8 (2009) PMCID: PMC2729365
Jin, R., Rummel, A., Binz, T. & Brunger, A.T. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 444:1092-5 (2006)
Jin, R., Clark, S., Weeks, A.M., Dudman, J.T., Gouaux, E. & Partin, K.M. Mechanism of positive allosteric modulators acting on AMPA receptors. J. Neurosci. 25(39):9027-36 (2005)
Jin, R., Junutula, J.R., Matern, H.T., Ervin, K.E., Scheller, R.H. & Brunger, A.T. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 24:2064-74 (2005)
Jin, R., Bank, T., Mayer, M. L., Traynelis, S. & Gouaux, E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 6(8):803-10 (2003)

Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. CHINA RONGSHENG HEAVY INDUSTRIES GROUP HOLDINGS LIMITED
The board of directors (the "Board") of the Company has noticed recent media reports that Mr. Wang Ping (who wholly owns and controls the Subscriber) has been detained by the Beijing police for investigation on matters which are not related to the Group or the Warrant Issue. The Company has no information as to the details of the incident and has been unable to contact Mr. Wang Ping, which casts doubt over the ability of the Subscriber to perform its obligations under the Subscription Agreement prior to the Long Stop Date of 31 March 2015. In light of the current circumstances, the Board has decided that it is not in the best interests of the Company and its shareholders to proceed with the Warrant Issue. The Company will seek legal advice on the arrangement regarding proposed resolution no. 1 as set out in the Notice of EGM to approve the Subscription Agreement, the Warrant Issue and the specific mandate to allot and issue the Subscription Shares. Further announcement(s) will be made by the Company as and when appropriate in respect of any material development on the above matters.
By Order of the Board China Rongsheng Heavy Industries Group Holdings Limited LEE Man Yee

The Board is pleased to announce that the English name of the Company has been changed from "China Rongsheng Heavy Industries Group Holdings Limited" to "China Huarong Energy Company Limited" and the Chinese name of the Company has been changed from
The stock short name of shares of the Company for trading on the Stock Exchange will be changed from "CH RONGSHENG" to "HUARONG ENERGY" in English and from "中國 熔盛重工" to "華榮能源" in Chinese with effect from 9:00 a.m. on 24 April 2015. The stock
Reference is made to the announcement of China Huarong Energy Company Limited (formerly known as China Rongsheng Heavy Industries Group Holdings Limited) (the "Company") dated 29 October 2014 and the circular of the Company dated 17 February
The Board is pleased to announce that the English name of the Company has been changed from "China Rongsheng Heavy Industries Group Holdings Limited" to "China Huarong Energy Company Limited" and the Chinese name of the Company has been changed from
The stock short name of shares of the Company for trading on the Stock Exchange will be changed from"CH RONGSHENG" to "HUARONG ENERGY" in English and from "中國熔 盛重工" to "華榮能源" in Chinese with effect from 9:00 a.m. on 24 April 2015. The stock
Citation:Lee K, Gu S, Jin L, Le TTN, Cheng LW, Strotmeier J, et al. (2013) Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLoS Pathog 9(10):
20.Inoue K, Fujinaga Y, Watanabe T, Ohyama T, Takeshi K, et al. (1996) Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun 64: 1589–1594.
25.Fujinaga Y, Inoue K, Nomura T, Sasaki J, Marvaud JC, et al. (2000) Identification and characterization of functional subunits of Clostridium botulinum type A progenitor toxin involved in binding to intestinal microvilli and erythrocytes. FEBS Lett 467: 179–183.
26.Inoue K, Fujinaga Y, Honke K, Arimitsu H, Mahmut N, et al. (2001) Clostridium botulinum type A haemagglutinin-positive progenitor toxin (HA(+)-PTX) binds to oligosaccharides containing Gal beta1-4GlcNAc through one subcomponent of haemagglutinin (HA1). Microbiology 147: 811–819.
27.Sugawara Y, Matsumura T, Takegahara Y, Jin Y, Tsukasaki Y, et al. (2010) Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J Cell Biol 189: 691–700.
28.Jin Y, Takegahara Y, Sugawara Y, Matsumura T, Fujinaga Y (2009) Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins - differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology 155: 35–45.
29.Matsumura T, Jin Y, Kabumoto Y, Takegahara Y, Oguma K, et al. (2008) The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol 10: 355–364.
32.Fujita R, Fujinaga Y, Inoue K, Nakajima H, Kumon H, et al. (1995) Molecular characterization of two forms of nontoxic-nonhemagglutinin components of Clostridium botulinum type A progenitor toxins. FEBS Lett 376: 41–44.
33.Ohyama T, Watanabe T, Fujinaga Y, Inoue K, Sunagawa H, et al. (1995) Characterization of nontoxic-nonhemagglutinin component of the two types of progenitor toxin (M and L) produced by Clostridium botulinum type D CB-16. Microbiol Immunol 39: 457–465.
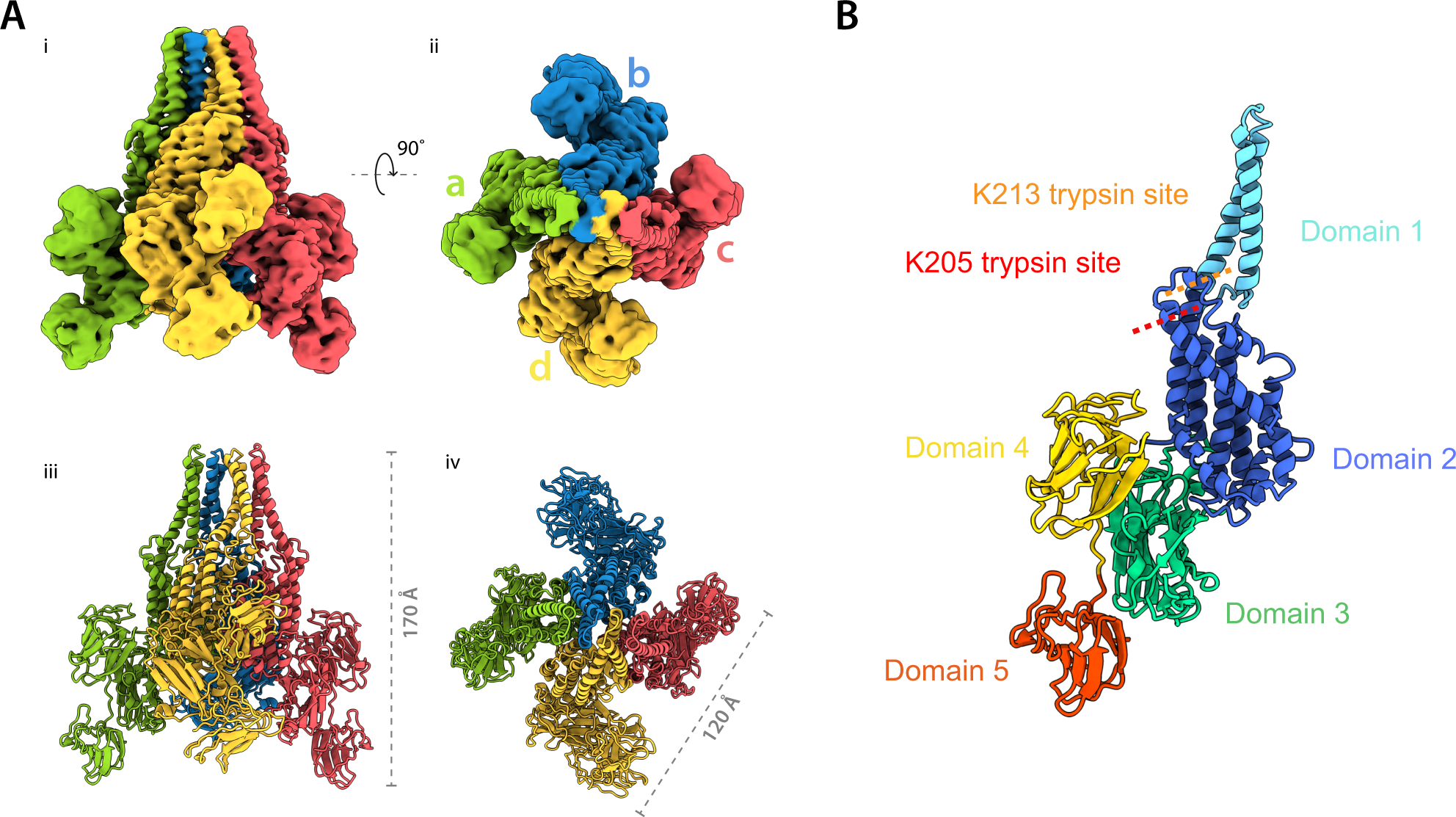
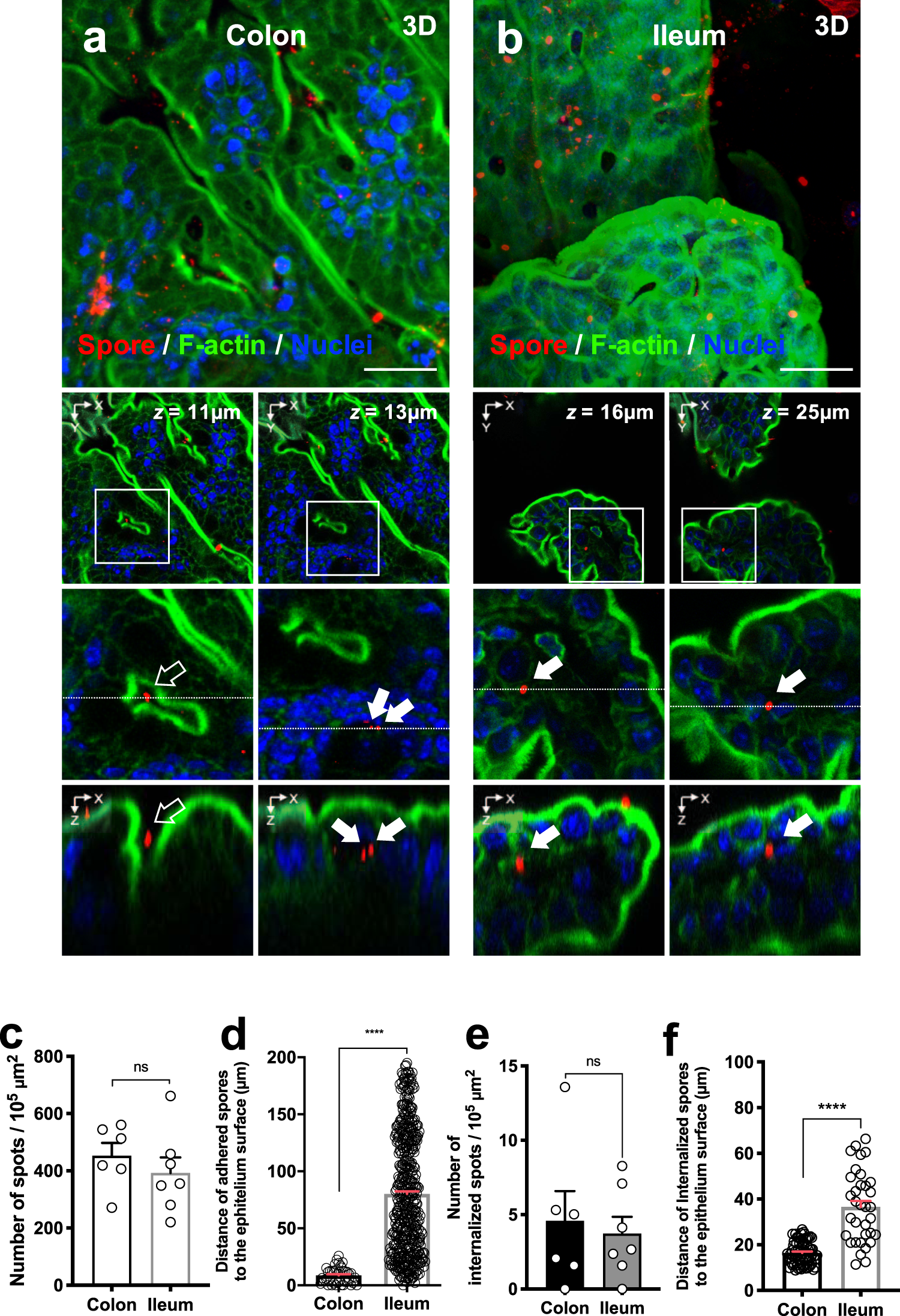
In foodborne botulism, BoNT must cross intestinal epithelial barriers to enter the blood stream and reach target neurons. The mechanism of how the large BoNT holotoxin or its multi-subunit complexes traverse the polarized epithelial monolayer is not fully understood. The three-dimensional structure of the BoNT/A complex has recently been elucidated (Gu et al., 2012; Lee et al., 2013). Carbohydrate binding sites have been identified and recently shown to be involved in binding to intestinal epithelia. BoNT holotoxins are known to cross the intestinal epithelium from the apical side and relocate to the basolateral side via transcytosis (Maksymowych and Simpson, 1998; 2004,; Ahsan et al., 2005; Fujinaga et al., 2009; Fujinaga, 2010). Large BoNT complexes have been shown to increase oral toxicity in mice by about 20 times over that of purified holotoxin. Toxin complex size is directly proportional to oral toxicity: the larger the complex, the greater the oral toxicity (Ohishi et al., 1977; Sugii et al., 1977; Chen et al., 1998). Studies by others suggest an active role for the NAPs, such as HA33 binding to surface receptors followed by internalization and transcytosis. HA33 was shown to disrupt epithelial tight junctions from the basolateral side, promoting rapid toxin complex passage via a paracellular mechanism (Fujinaga et al., 2009; Jin et al., 2009).
There are at least two proposed mechanisms of BoNT translocation. The first purports that NAPs only protect BoNTs from the low pH and degradative effects of intestinal juices and play no role in holotoxin uptake. In this model, BoNT holotoxin alone is able to traverse the intestinal and lung epithelium and reach the bloodstream intact (Maksymowych et al., 1999; Ahsan et al., 2005; Al-Saleem et al., 2012; Couesnon et al., 2012; Simpson, 2013). In a second model, NAPs play a direct function in binding intestinal receptors, disrupting tight junctions in the intestinal epithelial cell barrier, and promoting the paracellular transport of BoNTs following initial transcytosis (Matsumura et al., 2008; Jin et al., 2009; Sugawara et al., 2010). However, most of the evidence supporting either hypothesis is derived from in vitro cultured intestinal cell models and limited ex vivo studies that do not provide a complete picture of how toxin complexes translocate across the intestinal epithelia.
HA33, a component of the HA-C, was previously shown to disrupt membrane tight junctions (Fujinaga et al., 2009; Jin et al., 2009). However, these studies utilized unusual growth conditions such as low temperatures as well as very large amounts of toxins. To determine the effect of BoNT on epithelial cell membrane integrity and the role of the HA-C, we cultured polarized epithelial cells in transwells and measured the transepithelial electrical resistance (TER). A decrease in TER indicates a disruption in tight junctions. We applied either the BoNT/A holotoxin, the toxin complex, or the HA-C alone, or in combination with holotoxin, to the apical sides and measured TER values at 37°C under neutral pH over a period of 24 h. When polarized Caco-2 cells were treated with either the BoNT/A complex or the HA-C, the TER value dropped to approximately 55% after 10 h of exposure. This change in TER indicated a slight disruption of tight junctions as compared with the control, Hank’s Balanced Salt Solution (HBSS) or following treatment with BoNT/A holotoxin, where the TER dropped to approximately 86% after 10 h (Fig. 3A). No significant differences in TER values were observed in Caco-2 cells exposed to the holotoxin or the control media (Fig. 3A). Surprisingly, when Caco-2 cells were exposed basolaterally to the BoNT/A complex and HA-C, only a small decrease in TER value to 70% was observed. Likewise, a small decrease in TER to approximately 80% following exposure to BoNT/A holotoxin was observed, indicating minimal disruption of tight junctions (Fig. 3B). We also tested TER on cells treated with BoNT/A complex under acidic pH (pH 6.0) but did not observe any significant differences (data not shown).
What we know thus far from in vivo and ex vivo assays is that BoNT itself can transcytose across the intestinal epithelium (Fujinaga, 2010; Couesnon et al., 2012; Yao et al., 2014). Yet, there is a body of indirect and direct evidence which argues that the HA proteins in NAPs can interact with the intestinal epithelia. In one hypothesis, Fujinaga et al. proposed a three-step mechanism: (i) transcytosis, (ii) intestinal barrier disruption and (iii) entry of toxin through damaged epithelia (Fujinaga, 2010). The HAs bind mainly on the basolateral surface inducing loss of the paracellular barrier or cause cell damage, allowing toxin to move into the cells. HA33 was shown to facilitate transport of BoNT/D across epithelium and treatment with antibodies against HA70 and HA33 as well as incubation with sialic acid reduced cell binding and transport (Hasegawa et al., 2007). Recently, HA proteins were shown to interact with intestinal saccharides in a sialic acid or galactose-dependent process. Binding and transport were inhibited with anti-HA33 antibodies or the addition of saccharides (Ito et al., 2011). Although BoNT holotoxins alone can enter intestinal epithelial cells, NAPs play a role to facilitate its internalization.
What role do NAPs play in translocation? Previous data suggested that NAPs, specifically HA33, can disrupt tight junctions, compromising the epithelial cell barrier and allowing BoNT complex to enter through a paracellular pathway (Fujinaga, 2010). The HA-C was also shown to bind E-cadherin, a cell adhesion protein that likely plays a major role in toxin cell surface binding (Sugawara et al., 2010; Lee et al., 2014). The HA-C was observed to disrupt membrane integrity (measured by TER) quickly after toxin or HA addition and much faster after addition of toxins or HA to the basolateral side. These data combined with the location of E-cadherin in the intercellular space suggested that the paracellular translocation pathway was the likely route for toxin complex entry (Lee et al., 2014). In contrast, our results from measuring TER of polarized membranes of Caco-2 cell showed a gradual and mild disruption of membrane beginning at about 4 h after BoNT/A complex or HA-C addition to the apical side (Fig. 3A). When toxins were added to the basolateral side, an even smaller degree of membrane disruption was observed with BoNT/A complex compared with BoNT/A holotoxin (Fig. 3B). We did not observe obvious intestinal membrane disruptions in our small intestinal cross sections (Fig. 5). When BoNT/A holotoxin and the HA-C were added at the same time, no increase in the timing of BoNT/A holotoxin translocation to Caco-2 or small intestinal villi was observed (Fig. 2D and Supporting Information Fig. S5). Thus, in the absence of the NTNH link between the HA-C and BoNT/A holotoxin, toxin entry was not facilitated through a paracellular pathway of transport. The slight membrane disruption observed in TER reductions probably led to some paracellular translocation of the toxin but was likely not the main contributor to BoNT/A translocation. However, despite our results, we believe both models (paracellular vs. receptor-mediated internalization) are possible depending on the amount of toxins present. In the previous TER model, when in the presence of high levels of BoNT toxins, the predominant mode of transport is the likely paracellular pathway mediated by E-cadherin disruption of tight junctions. Our TER assays use substantially less toxin or HA protein than other assays and thus we do not observe the obvious effect of tight junction disruption.
Botulinum neurotoxin serotype A holotoxin, BoNT/A complex and rabbit polyclonal antibodies against BoNT/A were purchased from Metabiologics. The median lethal dose of BoNT/A holotoxin and complex was estimated at 0.42 ng kg−1 or about 8 pg/mouse by intraperitoneal injection and 27 μg kg−1 or about 0.5 μg/mouse for BoNT/A complex via oral gavage (Cheng et al., 2008). Caco-2, a human epithelial colon adenocarcinoma cell line, was purchased from the American Type Culture Collection. Chemicals and reagents were purchased from Sigma-Aldrich and tissue culture supplies were from Life Technologies. Monoclonal antibodies (mAbs): a mAb against the NTNH complex protein, referred to as NTNH 84-27-7-5, was generated following immunization with a recombinant protein corresponding to aa 616-1193 of the NTNH molecule and prepared in Phosphate Buffered Saline (PBS) by the Stanker laboratory (unpublished results); the HA70 specific mAb, NAP80-7-2-1 (Stanker et al., 2013). The recombinant HA-C was obtained from the Jin lab and was prepared as described in Lee et al. 2013 (Gu et al., 2012; Lee et al., 2013).
Fujinaga Y, Matsumura T, Jin Y, Takegahara Y. Sugawara Y. A novel function of botulinum toxin-associated proteins: HA proteins disrupt intestinal epithelial barrier to increase toxin absorption. Toxicon.2009;54:583–586. [PubMed]
Inoue K, Fujinaga Y, Watanabe T, Ohyama T, Takeshi K, Moriishi K, et al. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect Immun.1996;64:1589–1594. PubMed]
Jin Y, Takegahara Y, Sugawara Y, Matsumura T. Fujinaga Y. Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins – differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology.2009;155:35–45. [PubMed]
Lee K, Gu S, Jin L, Le TT, Cheng LW, Strotmeier J, et al. Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog.2013;9:e1003690. PubMed]
Matsumura T, Jin Y, Kabumoto Y, Takegahara Y, Oguma K, Lencer WI. Fujinaga Y. The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol.2008;10:355–364. [PubMed]
Sugawara Y, Matsumura T, Takegahara Y, Jin Y, Tsukasaki Y, Takeichi M. Fujinaga Y. Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J Cell Biol.2010;189:691–700. PubMed]
Yao G, Lee K, Gu S, Lam KH. Jin R. Botulinum neurotoxin A complex recognizes host carbohydrates through its hemagglutinin component. Toxins.2014;6:624–635. PubMed]




 8613371530291
8613371530291