hoffman rotary table quotation

Manual precision rotary table with compact and space saving design. The high rigidity allows the use for machine tools as well as for measuring applications.
Additional direct indexing plate for the indexing plate device for all divisions up to 100 and higher ones, many up to 400, with included adjusting table.

Hofmann Rotary Tables has developed a solid reputation for high-quality and affordable indexing devices, rotary tables, and tailstocks. Superior Spindle is proud to be a factory service partner for all, including the HPRS, HTH, R, and WR series.
Our Repair ProcessDisassembly & Free Evaluation: When you send your Hofmann rotary table to us, our team will fully disassemble it in our HEPA Class 10,000 cleanroom and evaluate all components for signs of wear and tear or damage.
Testing & Inspection: After repairs are complete, our team tests the rotary table to ensure optimal performance. Before your table is shipped back to you, our Quality Assurance team will perform a final inspection.
Shipping: All shipping and handling is covered by Superior Spindle. When you receive your newly refurbished table, we will provide you with a list of all components that were repaired/replaced as well as recommended maintenance.
Ourcutting-edge facility allows us to offer a wide array of additional services. Beyond our standard and expedited Hofmann rotary table repairs, we also provide vibration analysis, engineering recommendations, and retrofitting. Superior Spindle understands precision and is able to hold tolerances to 1 micron.
Superior Spindle has been a trusted provider of Hofmann rotary table repairs for over a decade and provides services to manufacturers throughout North America. If you’re experiencing issues with your rotary table, or are looking for design upgrade recommendations, call (734) 224-4778 orfill out our online formtoday.
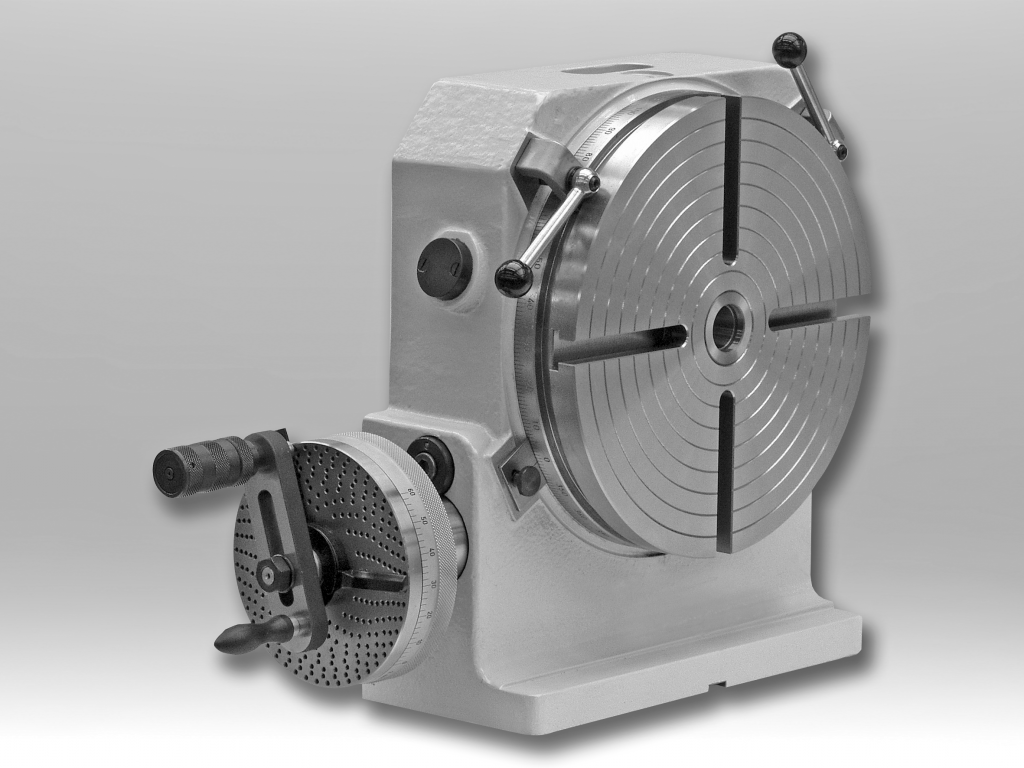
Angled Rotary Table WR is a manual precision angled rotary tables in a compact, space-saving design with high rigidity have been designed for use with horizontal or vertical turning axis on machine tools or for measuring tasks. Its available in the types WR, WRI, WRD und WRDI .Rotary Tables RH are Manual Precision Rotary Tables with compact and space saving design. The high rigidity allows the use for machine tools as well as for measuring applications. Table plate has 200 mm diameter. The table is available in the types RH and RHI.

When space allows, we use a rotary compressor because they historically last longer and are more energy-efficient. Our TXV’s are exclusively Danfoss because they are the only TXV manufacturer that uses a stainless steel outside and inside copper blend for durability.
Pfeiffer Helium Test - As the smallest particle in the periodic table, smaller than the particles in our r410a refrigerant, helium is uniquely qualified to find any microscopic holes. While pressurized at 150 PSI a helium “sniffer” traces the entire line to look for any traces of a leak.

A rotary table has no stops so it is not convenient to do large numbers of things at equal intervals because you would have to painstakingly determine the interval. Also, the rotary table does not divide the circle. For example, if you were making 13 equally spaced operations using a rotary table you would have to calculate some wierd angle for each operation and dial it in--a tedious process. For example, here are the 13 angles for a circle division:
The Woodward-Hoffmann rules in action: Thermolysis of 1 yields the (E,E) geometric isomer 2, whereas thermolysis of 3 yields the (E,Z) geometric isomer 4.
The Woodward–Hoffmann rules (or the pericyclic selection rules),Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reactions, an important class of reactions in organic chemistry. The rules are best understood in terms of the concept of the conservation of orbital symmetry using orbital correlation diagrams (see Section 3 below). The Woodward–Hoffmann rules are a consequence of the changes in electronic structure that occur during a pericyclic reaction and are predicated on the phasing of the interacting molecular orbitals. They are applicable to all classes of pericyclic reactions (and their microscopic reverse "retro" processes), including (1) electrocyclizations, (2) cycloadditions, (3) sigmatropic reactions, (4) group transfer reactions, (5) ene reactions,cheletropic reactions,dyotropic reactions.molecular orbital theory to experimental chemists.
Woodward and Hoffmann developed the pericyclic selection rules by examining correlations between reactant and product orbitals (i.e., how reactant and product orbitals are related to each other by continuous geometric distortions that are functions of the reaction coordinate). They identified the conservation of orbital symmetry as a crucial theoretical principle that dictates the outcome (or feasibility) of a pericyclic process. Other theoretical approaches that lead to the same selection rules have also been advanced. Hoffmann was awarded the 1981 Nobel Prize in Chemistry for elucidating the importance of orbital symmetry in pericyclic reactions, which he shared with Kenichi Fukui. Fukui developed a similar set of ideas within the framework of frontier molecular orbital (FMO) theory. Because Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize in Chemistry.
A pericyclic reaction is an organic reaction that proceeds via a single concerted and cyclic transition state, the geometry of which allows for the continuous overlap of a cycle of (π and/or σ) orbitals. In the language of orbital symmetry, a pericyclic reaction is termed symmetry-forbidden if there is an additional symmetry-imposed energetic barrier arising from the intended correlation of the ground state electron configuration of the starting material with an excited state electron configuration of the product and vice versa. (Although the non-crossing rule forbids such a correlation, the rise in energy as the intended crossing is approached results in an additional energy barrier nonetheless.) A pericyclic reaction is classified as symmetry-allowed if no such symmetry-imposed barrier exists. Thus, these terms do not imply whether a reaction in question will actually take place. Rather, with all other energetic factors being equal, a symmetry-forbidden process will be impeded by an additional energetic barrier. Although the symmetry-imposed barrier is often formidable (up to ca. 5 eV or 480 kJ/mol in the case of a forbidden [2+2] cycloaddition), the prohibition is not absolute, and symmetry-forbidden reactions can still take place via a pericyclic pathway if other factors (e.g. strain release) favor the reaction. Likewise, a symmetry-allowed reaction may be preempted by an insurmountable energetic barrier resulting from factors unrelated to orbital symmetry.
The Woodward–Hoffmann rules were first formulated in 1965 to explain the striking stereospecificity of electrocyclic reactions under thermal and photochemical control. The stereochemistry of electrocyclization was synthetically important in the context of Woodward"s longstanding endeavor to synthesize vitamin B12, and observations made in the course of the synthesis served to inspire the formulation of the Woodward–Hoffmann rules. The interconversion of model cyclobutene and butadiene derivatives under thermal (heating) and photochemical (Ultraviolet irradiation) conditions is illustrative.Thermolysis of trans-1,2,3,4-tetramethyl-1-cyclobutene (1) afforded only one geometric isomer, (E,E)-3,4-dimethyl-2,4-hexadiene (2); the (Z,Z) and the (E,Z) geometric isomers were not detected in the product mixture. Similarly, thermolysis of cis-1,2,3,4-tetramethyl-1-cyclobutene (3) afforded only (E,Z) isomer 4.same direction.E,E)-2,4-hexadiene (5) was exposed to light, cis-3,4-dimethyl-1-cyclobutene (6) was formed exclusively as a result of electrocyclic ring closure.opposite directions to form the new σ-bond. Thermolysis of 6 follows the same stereochemical course as 3: electrocyclic ring opening leads to the formation of (E,Z)-2,4-hexadiene (7) and not 5.
The terms conrotatory and disrotatory were coined to describe the relative sense of bond rotation involved in electrocyclic ring opening and closing reactions. When the two ends of the breaking or forming bond rotate in the same direction (both clockwise or both counterclockwise — as in the case of the ring opening of 1, 3 or 6 under thermal conditions), the process is termed conrotatory. When the two ends rotate in opposing directions (one clockwise, one counterclockwise — as in the photochemical ring closing of 5), the process is termed disrotatory. It was found that 4n-electron thermal and (4n + 2)-electron photochemical electrocyclic reactions were conrotatory in general, while 4n-electron photochemical and (4n + 2)-electron thermal electrocyclic reactions were disrotatory in general. This pattern was first explained in 1965, when Woodward and Hoffmann proposed the conservation of orbital symmetry (see below) as a key principle that governs the stereochemical course of electrocyclic reactions.
Eventually, it was recognized that thermally-promoted pericyclic reactions in general obey a single set of generalized selection rules, depending on the electron count and topology of the orbital interactions. The key concept of orbital topology or faciality was introduced to unify several classes of pericyclic reactions under a single conceptual framework. In short, a set of contiguous atoms and their associated orbitals that react as one unit in a pericyclic reaction is known as a component, and each component is said to be suprafacial depending on whether the orbital lobes that interact during the reaction are on the opposite or same side of the nodal plane, respectively. (The older terms conrotatory and disrotatory, which are applicable to electrocyclic ring opening and closing only, are subsumed by the terms antarafacial and suprafacial, respectively, under this more general classification system.) Given these general definitions, the Woodward–Hoffmann rules can be stated succinctly as a single sentence:Generalized pericyclic selection rule. A ground-state pericyclic process involving N electron pairs and A antarafacial components is symmetry-allowed if and only if N + A is odd.
Between the initial development of the principle of orbital symmetry conservation in 1965 by Woodward and Hoffmann and their statement of the generalized pericyclic selection rule in 1969, Howard ZimmermanMichael J. S. DewarMöbius-Hückel concept, or aromatic transition state theory to explain the reactivity and selectivity of pericyclic systems, while Kenichi Fukuifrontier orbital theory. In the Dewar-Zimmerman approach the topology of orbital overlap (Hückel or Möbius) and the total electron count of the system (4n + 2 or 4n) result in transition states that are classed as either aromatic or antiaromatic. In the language of aromatic transition state theory, the Woodward–Hoffmann rules can be restated as follows: A pericyclic transition state involving (4n + 2) electrons with Hückel topology or 4n electrons with Möbius topology is aromatic and allowed, while a pericyclic transition state involving 4n-electrons with Hückel topology or (4n + 2)-electrons with Möbius topology is antiaromatic and forbidden. The Fukui approach, on the other hand, analyzes the interactions between the HOMO and LUMO of each of the reactants, or within a reactant. A process in which the HOMO-LUMO interaction is constructive (results in a net bonding interaction) is favorable and considered symmetry-allowed, while a process in which the HOMO-LUMO interaction is non-constructive (results in bonding and antibonding interactions that cancel) is disfavorable and considered symmetry-forbidden. The correlation diagram approach (the conservation of orbital symmetry, vide supra), as proposed by Woodward and Hoffmann and clarified by Longuet-Higgins and others, led to the general statement that a pericyclic reaction is allowed if the sum of the number of suprafacial 4q + 2 components and number of antarafacial 4r components is odd. Importantly, though conceptually distinct, aromatic transition state theory (Zimmerman and Dewar), frontier molecular orbital theory (Fukui), and the principle of orbital symmetry conservation (Woodward and Hoffmann) make identical predictions.
The Woodward–Hoffmann rules were first invoked to explain the observed stereospecificity of electrocyclic ring-opening and ring-closing reactions at the ends of open chain conjugated polyenes either by application of heat (thermal reactions) or application of light (photochemical reactions).
As shown by Longuet-Higgins and E. W. Abrahamson, the Woodward–Hoffmann rules can best be derived by examining the correlation diagram of a given reaction.symmetry element is a point of reference (usually a plane or a line) about which an object is symmetric with respect to a symmetry operation. If a symmetry element is present throughout the reaction mechanism (reactant, transition state, and product), it is called a conserved symmetry element. Then, throughout the reaction, the symmetry of molecular orbitals with respect to this element must be conserved. That is, molecular orbitals that are symmetric with respect to the symmetry element in the starting material must be correlated to (transform into) orbitals symmetric with respect to that element in the product. Conversely, the same statement holds for antisymmetry with respect to a conserved symmetry element. A molecular orbital correlation diagram correlates molecular orbitals of the starting materials and the product based upon conservation of symmetry. From a molecular orbital correlation diagram one can construct an electronic state correlation diagram that correlates electronic states (i.e. ground state, and excited states) of the reactants with electronic states of the products. Correlation diagrams can then be used to predict the height of transition state barriers.
The Woodward–Hoffmann rules can also explain bimolecular cycloaddition reactions through correlation diagrams.πp + πq] cycloaddition brings together two components, one with p π-electrons, and the other with q π-electrons. Cycloaddition reactions are further characterized as suprafacial (s) or antarafacial (a) with respect to each of the π components. (See below "General formulation" for a detailed description of the generalization of WH notation to all pericyclic processes.)
Using correlation diagrams one can derive selection rules for the following generalized classes of pericyclic reactions. Each of these particular classes is further generalized in the generalized Woodward–Hoffmann rules. The more inclusive bond topology descriptors antarafacial and suprafacial subsume the terms conrotatory and disrotatory, respectively. Antarafacial refers to bond making or breaking through the opposite face of a π system, p orbital, or σ bond, while suprafacial refers to the process occurring through the same face. A suprafacial transformation at a chiral center preserves stereochemistry, whereas an antarafacial transformation reverses stereochemistry.
The selection rule of electrocyclization reactions is given in the original statement of the Woodward–Hoffmann rules. If a generalized electrocyclic ring closure occurs in a polyene of 4n π-electrons, then it is conrotatory under thermal conditions and disrotatory under photochemical conditions. Conversely in a polyene of 4n + 2 π-electrons, an electrocyclic ring closure is disrotatory under thermal conditions and conrotatory under photochemical conditions.
The ene reaction is often classified as a type of group transfer process, even though it does not involve the transfer of two σ-bonded groups. Rather, only one σ-bond is transferred while a second σ-bond is formed from a broken π-bond. As an all suprafacial process involving 6 electrons, it is symmetry-allowed under thermal conditions. The Woodward-Hoffmann symbol for the ene reaction is [π2s + π2s + σ2s] (see below).
Though the Woodward–Hoffmann rules were first stated in terms of electrocyclic processes, they were eventually generalized to all pericyclic reactions, as the similarity and patterns in the above selection rules should indicate.
In the generalized Woodward–Hoffmann rules, everything is characterized in terms of antarafacial and suprafacial bond topologies. The terms conrotatory and disrotatory are sufficient for describing the relative sense of bond rotation in electrocyclic ring closing or opening reactions, as illustrated on the right. However, they are unsuitable for describing the topologies of bond forming and breaking taking place in a general pericyclic reaction. As described in detail below, in the general formulation of the Woodward–Hoffmann rules, the bond rotation terms conrotatory and disrotatory are subsumed by the bond topology (or faciality) terms antarafacial and suprafacial, respectively. These descriptors can be used to characterize the topology of the bond forming and breaking that takes place in any pericyclic process.
The generalized statement of the Woodward–Hoffmann rules states that a + d is odd if the reaction is allowed. Now, if a is even, then this implies that d is odd. Since b is odd in this case, the number of antarafacial components, b + d, is even. Likewise, if a is odd, then d is even. Since b even in this case, the number of antarafacial components, b + d, is again even. Thus, regardless of the initial assumption of parity for a and b, the number of antarafacial components is even when the electron count is 4n + 2. Contrariwise, in the forbidden case, where a + d is even, we can show that, regardless of initial assumptions, b + d is odd.
Finally, to complete the argument, and show that this new criterion is truly equivalent to the original criterion, one needs to argue the converse statements as well, namely, that the number of antarafacial components b + d and the electron count (4n + 2 or 4n) implies the parity of a + d that is given by the Woodward–Hoffmann rules (odd for allowed, even for forbidden). Another round of (somewhat tedious) case analyses will easily show this to be the case.
In this formulation, the electron count refers to the entire reacting system, rather than to individual components, as enumerated in Woodward and Hoffmann"s original statement. In practice, an even or odd number of antarafacial components usually means zero or one antarafacial components, respectively, as transition states involving two or more antarafacial components are typically disfavored by strain. As exceptions, certain intramolecular reactions may be geometrically constrained in such a way that enforces an antarafacial trajectory for multiple components. In addition, in some cases, e.g., the Cope rearrangement, the same (not necessarily strained) transition state geometry can be considered to contain two supra or two antara π components, depending on how one draws the connections between orbital lobes. (This ambiguity is a consequence of the convention that overlap of either both interior or both exterior lobes of a σ component can be considered to be suprafacial.)
This alternative formulation makes the equivalence of the Woodward–Hoffmann rules to the Dewar–Zimmerman analysis (see below) clear. An even total number of phase inversions is equivalent to an even number of antarafacial components and corresponds to Hückel topology, requiring 4n + 2 electrons for aromaticity, while an odd total number of phase inversions is equivalent to an odd number of antarafacial components and corresponds to Möbius topology, requiring 4n electrons for aromaticity.Thermal pericyclic reactions proceed via (4n + 2)-electron Hückel or (4n)-electron Möbius transition states.
If the total number of electrons is 4n + 2, then one is in the bottom row of the table. The reaction is thermally allowed if it is suprafacial with respect to both components or antarafacial with respect to both components. That is to say the number of antarafacial components is even (it is 0 or 2). Similarly if the total number of electrons is 4n, then one is in the top row of the table. This is thermally allowed if it is suprafacial with respect to one component and antarafacial with respect to the other. Thus the total number of antarafacial components is always odd as it is always 1.
The generalized Woodward–Hoffmann rules, first given in 1969, are equivalent to an earlier general approach, the aromatic transition state theory.n electrons, the Möbius topology is aromatic and the Hückel topology is antiaromatic, while if the transition state involves 4n + 2 electrons, the Hückel topology is aromatic and the Möbius topology is antiaromatic. The parity of the number of phase inversions (described in detail below) in the transition state determines its topology. A Möbius topology involves an odd number of phase inversions whereas a Hückel topology involves an even number of phase inversions.
In connection with Woodward–Hoffmann terminology, the number of antarafacial components and the number of phase inversions always have the same parity.n-electron reaction is thermally allowed if and only if it has an odd number of antarafacial components (i.e., Möbius topology); a (4n + 2)-electron reaction is thermally allowed if and only if it has an even number of antarafacial components (i.e., Hückel topology).
Importantly, any scheme of assigning relative phases to the basis orbitals is acceptable, as inverting the phase of any single orbital adds 0 or ±2 phase inversions to the total, an even number, so that the parity of the number of inversions (number of inversions modulo 2) is unchanged.
The Woodward–Hoffmann rules are reinterpreted using this formulation by matching favorable interactions between regions of electron density for which the dual descriptor has opposite signs. This is equivalent to maximizing predicted favorable interactions and minimizing repulsive interactions. For the case of a [4+2] cycloaddition, a simplified schematic of the reactants with the dual descriptor function colored (red=positive, blue=negative) is shown in the optimal supra/supra configuration to the left. This method correctly predicts the WH rules for the major classes of pericyclic reactions.
In Chapter 12 of The Conservation of Orbital Symmetry, entitled "Violations," Woodward and Hoffmann famously stated:There are none! Nor can violations be expected of so fundamental a principle of maximum bonding.
This pronouncement notwithstanding, it is important to recognize that the Woodward–Hoffmann rules are used to predict relative barrier heights, and thus likely reaction mechanisms, and that they only take into account barriers due to conservation of orbital symmetry. Thus it is not guaranteed that a WH symmetry-allowed reaction actually takes place in a facile manner. Conversely, it is possible, upon enough energetic input, to achieve an anti-Woodward-Hoffmann product. This is especially prevalent in sterically constrained systems, where the WH-product has an added steric barrier to overcome. For example, in the electrocyclic ring-opening of the dimethylbicyclo[0.2.3]heptene derivative (1), a conrotatory mechanism is not possible due to resulting angle strain and the reaction proceeds slowly through a disrotatory mechanism at 400o C to give a cycloheptadiene product.dioxetane-1,2-dione to two molecules of carbon dioxide, famous for its role in the luminescence of glowsticks, has been scrutinized computationally. In the absence of fluorescers, the reaction is now believed to proceed in a concerted (though asynchronous) fashion, via a retro-[2+2]-cycloaddition that formally violates the Woodward–Hoffmann rules.
Similarly, a recent paper describes how mechanical stress can be used to reshape chemical reaction pathways to lead to products that apparently violate Woodward–Hoffman rules.anti-WH product is predicted to be formed.
In a 2004 rebuttal published in the Roald Hoffmann denied the claim: he quotes Woodward from a lecture given in 1966 saying: "I REMEMBER very clearly—and it still surprises me somewhat—that the crucial flash of enlightenment came to me in algebraic, rather than in pictorial or geometric form. Out of the blue, it occurred to me that the coefficients of the terminal terms in the mathematical expression representing the highest occupied molecular orbital of butadiene were of opposite sign, while those of the corresponding expression for hexatriene possessed the same sign. From here it was but a short step to the geometric, and more obviously chemically relevant, view that in the internal cyclisation of a diene, the top face of one terminal atom should attack the bottom face of the other, while in the triene case, the formation of a new bond should involve the top (or pari passu, the bottom) faces of both terminal atoms."
In addition, Hoffmann points out that in two publications from 1963total synthesis of the compound dihydrocostunolide. Although they describe an electrocyclic reaction, Corey has nothing to offer with respect to explaining the stereospecificity of the synthesis.
The principle of orbital symmetry conservation is generally credited to Robert Burns Woodward and Roald Hoffmann, who proposed orbital symmetry conservation as an explanation for the stereochemical outcome of electrocyclic reactions (J. Am. Chem. Soc. 1965, 87, 395) and articulated a fully generalized pericyclic selection rule several years later (Angew. Chem. Int. Ed. Engl. 1969, 8, 781). However, E. J. Corey has claimed priority in proposing the key insight in 1965 (see "Controversy" section below). Moreover, E. Havinga had previously noted that tachysterol underwent electrocyclic ring closing in a conrotatory or disrotatory manner depending on activation mode (photochemical or thermal, respectively) and attributed an orbital symmetry explanation for this phenomenon to L. J. Oosterhoff (Tetrahedron Lett. 1961, 16, 146). In addition, aromatic transition state theory, advanced by H. E. Zimmerman (J. Am. Chem. Soc. 1966, 88, 1564) and M. J. S. Dewar (Tetrahedron 1966, Suppl. 8, 75), has been recognized as an alternative approach that is completely equivalent to, but predates, Woodward and Hoffmann"s statement of the generalized rule.
Geerlings, Paul; Ayers, Paul W.; Toro-Labbé, Alejandro; Chattaraj, Pratim K.; De Proft, Frank (2012). "The Woodward–Hoffmann Rules Reinterpreted by Conceptual Density Functional Theory". Accounts of Chemical Research. 45 (5): 683–95. doi:10.1021/ar200192t. hdl:PMID 22283422.
The Woodward–Hoffmann rules apply to either direction of a pericyclic process. Due to the inherent ring strain of cyclobutene derivatives, the equilibrium between the cyclobutene and the 1,3-butadiene lies far to the right. Hence, under thermal conditions, the ring opening of the cyclobutene to the 1,3-butadiene is strongly favored by thermodynamics. On the other hand, under irradiation by ultraviolet light, a
Woodward, R. B.; Hoffmann, Roald (1965). "Stereochemistry of Electrocyclic Reactions". Journal of the American Chemical Society. 87 (2): 395. doi:10.1021/ja01080a054.
Woodward, R. B.; Hoffmann, Roald (1971). The Conservation of Orbital Symmetry (3rd printing, 1st ed.). Weinheim, BRD: Verlag Chemie GmbH (BRD) and Academic Press (USA). pp. 1–178. ISBN 978-1483256153.
Hoffmann, Roald; Woodward, R. B. (1965). "Selection Rules for Concerted Cycloaddition Reactions". J. Am. Chem. Soc. 87 (9): 2046. doi:10.1021/ja01087a034.
Ayers, Paul W.; Morell, Christophe; De Proft, Frank; Geerlings, Paul (5 October 2007). "Understanding the Woodward–Hoffmann Rules by Using Changes in Electron Density". Chemistry: A European Journal. 13 (29): 8240–8247. doi:10.1002/chem.200700365. PMID 17639522.

AERATION of wastewater, Agitation of plating tanks, Air curtains for furnaces, Air flotation table, Air conditioning booster blowers, Air slide systems, Air dryers, Air knife.
TEMPERATURE (high) applications for air or gas up to 700 degrees F with special modifications, Tank purge systems breweries , etc., Tempering glass blowers, Tape removal systems, Tablet press pickup systems.

Dealing with insurance issues, for me, is just above dental issues on the scale of misery. Fortunately for me and my family we found Hoffman Insurance Group. I am self employed and my wife was also, and in getting us set up with insurance I needed extra help and guidance. (I"m a "special needs" insured, heh). HIG explained the options between the different carriers and gave some recommendations, pointing out the pros and cons between all the plans. How glad we were for HIG"s help when we found out in August 2011 my wife had breast cancer. Because of the assistance we"d received from Crystal Hoffman in 2008, we were not faced with catastrophic medical bills during the chemo and surgery ( and my wife has made a fully recovery!). We are grateful to HIG in helping us be prepared, and for their ongoing help as laws, etc. change. I can"t recommend Hoffman Insurange Group enough.

Wall-mount Solar Shield protects outdoor enclosures from overheating and provides weather protection from sun, rain and snow. Enclosures are shielded from the top, sides and rear; white paint finish maximizes solar heat reduction. Two sets of fully-adjustable mounting channels are provided to mount any type of wall-mount enclosure that fits inside the solar shield. Fits most Concept, InLine and NEMA Type 4 wall-mount enclosures and junction boxes (i.e. Bulletin A51NF and A51FL)

While vice president of the Rotary Interact club, she assisted with basketball concession sales, and selling baked goods to fundraise for Camp Nejeda, a camp for children with disabilities. Gabriella has taken part in planning events such as Relay for Life and the ARC’s Camp Sun Fun 5k. This year a major project will be the Leukemia and Lymphoma Student of the Year Campaign. This program is a fundraising competition in which nominated students compete over the course of six weeks in honor of a local teen patient battling cancer. Gabriella’s efforts, along with those of her fellow competitors, will directly benefit LLS’s National Capital Area Chapter.
In addition to her work with Rotary Interact and LLS, Gabriella is a member of the following extra curricular organizations: the Gay-Straight Alliance, Philosophy Club, Science Club, Mock Trial and the Outdoors Club. Thank you for your service Gabriella!
Several local fundraisers he’s participated in include the Buddy Walk, MS walk, Camp Sun N Fun, Relay for Life, and the Autumn Pasquale 5k Run for HOPE. International organizations such as Rotary’s Polio eradication, 4 South Sudan, and Oxfam Unwrapped have also been the beneficiaries of Isaiah’s fundraisers.
This past summer, Glassboro High School sent Isaiah to represent the school at RYLA (Rotary Youth Leadership Award) and the American Legion’s Boys State program. At Glassboro, Isaiah is president of his school engineering club and is a member of Mock Trial and the Interact club. Recently, he has been awarded the President’s Volunteer Service Award. Great job Isaiah!
Olivia also volunteers with the National Honor Society and Tri-M Music Honor Society. Through these organizations, she helped plan Township Makes Cut, an event that lets volunteers donate their hair to cancer patients, and perform for community members at a charity event. Additionally, she participated in the 2016 Rotary Youth Leadership Conference, and the 2016 EntrePrep Summer Institute. You go Olivia!
The law firm of Hoffman DiMuzio has allocated close to half a million dollars to its “Gift of the Heart” Community Scholarship Foundation. Forty-one graduating seniors from Gloucester and Salem County High Schools have been selected to receive a $1,000 scholarship for embodying the service ideals of the program. This week, the 2017 “Gift of the Heart” Hoffman DiMuzio Community Service Foundation recipient is from Our Lady of Mercy Academy (OLMA) — Dana Durham.

Great representation for WGA’s “now and future” at the DBW 4Sight orientation at City Year Jax. This generation doesn’t want to sit at the kids’ table. They want an inter-generational network for the greatest impact! Finding all that and more in WGA! Yay!!




 8613371530291
8613371530291